| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| charcoal fuel | ||
|
||
|
May 2014 Summary of charcoal as a fuel:
Charcoal is an engineered form of wood that is as old as human history. At its most primal, charcoal is simply organic matter like wood, grass and even bone heated in the absence of oxygen. Known as "pyrolysis", intense heat drives off most of the complex organic molecules as a vapor, leaving behind pure carbon and small quantities of minerals.
Without the tarry residues of wood, charcoal burns cleanly, and found use stoking pottery kilns and refining ore into metal. But charcoal production methods saw little innovation from the dawn of agriculture to the 1800s. Logs would be stacked into piles, covered with soil to block air, and burned gently through a hole dug in the top of the mound. Some of the wood provided heat, while the rest turned into charcoal.
Wood was also becoming scarce as a result of the industrial revolution, while sawdust and other industrial byproducts multiplied exponentially. Innovators converted this new "resource" into charcoal. One of the first to fabricate a charcoal briquette made out of compressed pyrolized sawdust was Ellsworth Zwoyer of Pennsylvania in 1897.
While briquettes were first invented as a low-cost fuel, it soon became obvious that a briquette might outperform lump charcoal on a number of critical dimensions. For example, the burn rate was more consistent, the temperature more controlled (and often higher than with lump), the odor minimized, and the dimensions perfected. Briquette manufacturing recipes are a closely held trade secret. They generally include some kind of binder, typically a starch, to glue the charcoal dust together into a mass. Limestone2 is added to control ashing and reduce sulfur emissions. Borax and sodium nitrate to even out the burn rate. Powdered coal for additional burn lifetime. Waxes to help the mass release from the briquette press. Truly an engineered3 product! Many vendors will call this brew "natural", since chemicals like borax are mined out of the earth. No different than carrots growing out of a field, I suppose. Marketing hype aside, charcoal briquettes allowed backyard cooks to build a reliable and consistent fire, and the grilling revolution began.
Because most of the organic chemicals were driven off by pyrolysis (and these chemicals produce the iconic smoke flavor), cooks usually add a few hunks of wood to the coal fire, as this cross-section of an *optimistically* filled smoker indicates. The wood adds flavor, while the coals provide heat and enhance the smoke ring. In wood fires (but not electric or gas), the combustion temperature varies widely across the coal tray. Some areas are hot and well ventilated producing sweet blue smoke, while others are puffing out smoldering white or gray smoke. Or even emitting black, bitter soot. A few coals are unlit, while others are covered with oxygen-blocking ash. Which means the fire is haphazardly refining cellulose and lignin into hundreds of chemicals, some desired and some not. If even a fraction of the fire is smoldering and cold, those bitter chemicals can overwhelm the sweeter flavors from hot wood. Thus good fire management is key in a charcoal smoker. Pitmasters in some parts of the country first convert wood into charcoal in a "burn barrel", and then shovel the charcoal into the smoker. Since they are only burning deep red coals, this method is more uniform and controllable -- if a bit tedious.
Avoid adding wet or green wood to the coals- their moisture cools the fire and cold smoke is bitter, though abundant. While "ideal" smoke flavor is a matter of taste (literally and figuratively), once you've tried clean smoke from a hot wood fire, the inadequacies of foggy gray smoke are apparent. Finally, there is the matter of air flow. Like wood combustion, charcoal combustion requires sufficient air to maintain average fire temperatures around 700F. Roughly speaking, you need 1 cf/hr (cubic feet per hour) of air for every 50 BTU/hr of wood fuel consumption. Charcoal energy density can be as high as 20 MJ/kg=5 KWhr/kg=8600 BTU/lb, but charcoal combustion is not as complete as with gas or electric. So 5000 BTU/lb is more realistic.
Every smoker is different, but I use about a pound of briquettes an hour. So 5000 BTU/hr demands 5000 BTU/hr/50 cf/BTU-hr=100 ft3/hr. A typical smoker might have an internal volume of 10 ft3, so the airflow is nearly 100 ft3/10 ft3=10 air exchanges an hour! And that is assuming all the air passes through the coals- in many smokers, most of the air never touches the flame. I typically measure 25-50 air exchanges an hour in most charcoal smokers. This high airflow rate may quickly dry out the meat's surface, reducing smoke adhesion and attraction. So a water pan, right above the fire where it can nearly boil, can restore humidity levels. Or a mop. Or wrap in the Texas crutch part way though cooking. A smoker which drops the briquettes next to the vent, in small quantities at a time, like some gravity fed smokers, can burn more efficiently since the air is where it is needed most. Bring the wood to the air, not the air to the wood! Unfortunately, the average smoker has a primitive vent arrangement, leading to common complaints about "bitter, over-smoked" meat. Even with the best of engineered fuels, there remains an important role for the pitmaster.
|
||
|
1 The Naked Wiz's website is an excellent source of product reviews for lump and some charcoal briquettes. 2 The limestone also preferentially converts nitrogen in coal into nitric oxide, creating a deeper smoke ring. Most lump charcoal is not fully pyrolized. Partly, the manufacturer leaves some unburned lignin in for flavor. But, it turns out pure carbon is very hard to ignite- one reason smelters carry red hot ore in graphite carbon crucibles. A bit of oily residue also helps the fire get started with newspaper, instead of a blow torch. 3 In many ways, charcoal briquette recipes are like cigarettes. Which contain numerous chemical additives to make sure the ember does not self-extinguish, and the flame burns uniformly. This is one reason fires are so common when a smoker falls asleep with a lit cigarette- it continues to burn without puffing. Cigarettes are a burning wood fire in a tube, producing carbon monoxide along with other toxic chemicals. Most smoker's blood CO levels run 2%-4%, and will lead to long-term physiological damage.
|
||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |
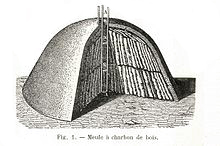 The first charcoals were probably the inadvertent byproduct of slash and burn agriculture. As flames danced across the fields, exposed wood and grasses were consumed by fire, but a few roots and logs, buried beneath the soil without enough oxygen to support combustion, simply heated and charred. This intensely black, remarkably lightweight "stone" dug from the ground would burn brightly when ignited in air. Eaten, it reduced stomach aches. Rubbed, it could be drawn across stone walls. Ground into paste, it became a black paint to decorate the human body.
The first charcoals were probably the inadvertent byproduct of slash and burn agriculture. As flames danced across the fields, exposed wood and grasses were consumed by fire, but a few roots and logs, buried beneath the soil without enough oxygen to support combustion, simply heated and charred. This intensely black, remarkably lightweight "stone" dug from the ground would burn brightly when ignited in air. Eaten, it reduced stomach aches. Rubbed, it could be drawn across stone walls. Ground into paste, it became a black paint to decorate the human body.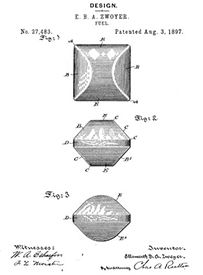 The problem with charcoal at the time (and even today when used for barbecue), is lack of consistency. Depending on the wood species, the heat of pyrolysis, seasoning level, etc "lump" charcoal might burn fast or slow, crackle and pop, smell like turpentine or burn clean and bright1. It might be dense or full of cracks. With high or low nitrogen content. And the shape is irregular.
The problem with charcoal at the time (and even today when used for barbecue), is lack of consistency. Depending on the wood species, the heat of pyrolysis, seasoning level, etc "lump" charcoal might burn fast or slow, crackle and pop, smell like turpentine or burn clean and bright1. It might be dense or full of cracks. With high or low nitrogen content. And the shape is irregular. In the 1920s Henry Ford and his cousin H.F. Kingston began manufacturing briquettes out of waste products from the manufacture of automobiles. Today, charcoal briquettes come in many sizes and shapes, from round to hexagonal to corrugated sheets. And can be pressed out of coconut or rice hulls, wood or bamboo.
In the 1920s Henry Ford and his cousin H.F. Kingston began manufacturing briquettes out of waste products from the manufacture of automobiles. Today, charcoal briquettes come in many sizes and shapes, from round to hexagonal to corrugated sheets. And can be pressed out of coconut or rice hulls, wood or bamboo. Some people arrange their briquettes in the shape of a long, sinuous maze (the "minion" method) lit from one end, so only a few coals are burning at one time. This lowers the temperature by reducing the size of the fire, rather than choking the air from a large tray of barely lit coals. Which is a step in the right direction. But the combustion air flows over the entire bed of briquettes, passing most of them by, so the burning edge is still oxygen starved. Many people brag about how long a half bag of coals can last; four, six hours- even overnight! But its not likely these barely combusting coals smell their best. Also, at low oxygen levels,
Some people arrange their briquettes in the shape of a long, sinuous maze (the "minion" method) lit from one end, so only a few coals are burning at one time. This lowers the temperature by reducing the size of the fire, rather than choking the air from a large tray of barely lit coals. Which is a step in the right direction. But the combustion air flows over the entire bed of briquettes, passing most of them by, so the burning edge is still oxygen starved. Many people brag about how long a half bag of coals can last; four, six hours- even overnight! But its not likely these barely combusting coals smell their best. Also, at low oxygen levels, 