| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| between a rock |
|
November 2011 Up until the last century or so, ovens were made from rock, clay or brick. They took hours to warm up, were hard to control, wasted limited fuels, and polluted our skies. Yet these ovens often produced great food with unique flavors and crusts. In the home today, ovens are constructed primarily from steel, baking cleanly with gas or electricity. But many people believe and hope lining their oven with ceramic stones (or their smokers with steel plates) might recapture these lost values. Once you've advanced from Pop n' Fresh dough to scratch made bread, its time to order a baking stone. Undeniably, the best surface to bake bread on is a flat hot stone. Instead of relying on the inefficient process of indirect heat transfer- from oven wall to warm air to bread- a loaf placed on a hot stone quickly heats up by direct conduction. Leading to a crisp bottom crust and often deep flavors. Everything from pizza beehive ovens to a tandoor clay pots relies on the magic of direct heat. But stones and related sources of thermal mass are reputed to offer the home cook additional advantages. If you place a hot rock in a well-sealed beer cooler, the interior will quickly rise up to match the rock's temperature. There will be few if any temperature fluctuations. Won't this effect apply in a kitchen oven? A quality frying pan's thermal conductivity evens out the gas flames heat supply- wouldn't this work in an oven? In all, I count five potential advantages of cooking with a stone or other thermal mass. They are:
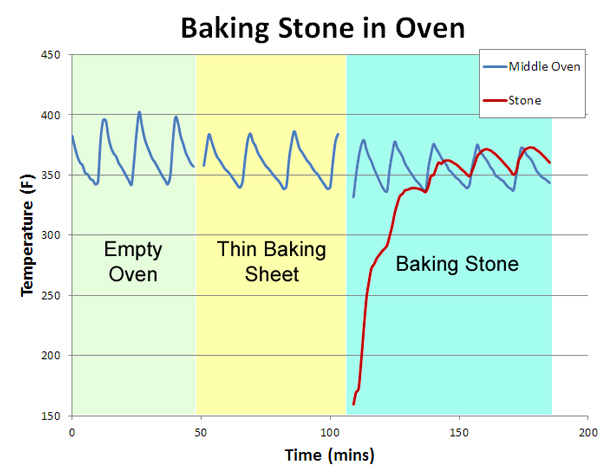
Modern ovens vary enormously in their uniformity. Our old electric oven was 50F hotter at the top than the bottom, and uneven from left to right. It leaked air like a sieve. Our new "professional commercial style" oven varies by only 5F from top to bottom and is very stable, but the temperature control knob is only accurate near 350F- it's off by 35F at the low end. And nearly all gas or electric oven's temperature varies in time, as the thermostat cycles on and off trying to maintain a fixed heat setting. We'll evaluate the effectiveness of a baking stone in three tests: an unmodified oven, an oven with a baking sheet to obscure uneven radiative heating from the red hot elements, and the standard home baking stone. Note the baking sheet and stone are both 14"x17", and placed on a rack immediately above the heating element:
The results are quite telling. In the empty oven (measured by a thermometer attached in the geometric center), the temperature cycles by +25F every ten minutes or so (the "sawtooth" blue curves below). Placing a thin aluminum baking sheet on the bottom shelf (a common suggestion in many cookbooks) does help, by blocking the direct thermal radiation of the red-hot elements. Now the temperature variation drops slightly to +20F. Finally, we add the baking stone (the red curve below- I drilled a hole into the center of the stone and inserted a thermocouple). Note it takes about an hour to come up to temperature. But, the net effect is under whelming. The oven temperature variations moderate a bit compared to cookie sheet alone- to perhaps +15F, but unlike the myth, the stone is not "rock solid", but cycles in parallel to the 1200F heating elements glowing an inch away.
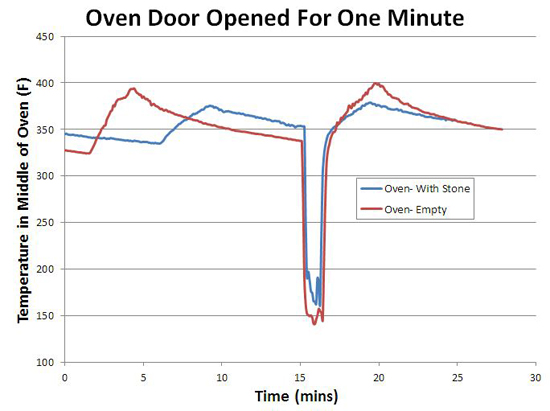
In other words, a typical baking stone in a typical oven does not even out thermal cycles, whether in the air or in the stone. If it did, the oven would cool off1 when the stone was inserted- but look again at this graph- the cold or warm stone had no effect on the air temperature! What about the other potential advantage of thermal mass- that is, to help the oven recover in temperature when the door is opened and closed? Again, pretty disappointing. The red curve illustrates an oven cycling along as normal, then the door is opened for one minute, then closed. The blue curve follows the same experimental protocol, with the baking stone in place. Note they both recover almost immediately!
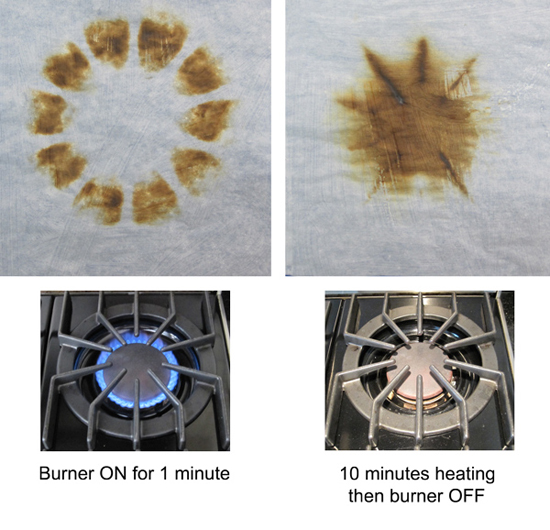
The reason is simple- the oven's thermostat detected the drop in temperature, and turned on the element in response, pulsing in a burst of compensating heat. Thermal mass, by itself, is "dumb", putting out the same amount of heat whether the door is ajar or not (see this article for more details)- pure thermal mass takes longer to recover. Another reason the modern oven, if properly designed, is potentially superior. Plus, the baking stone makes only a small contribution to the total thermal mass of a large steel oven. You can detect its influence, but it's not a dominant effect. Right now we are batting "1 for 4". The baking stone unequivocally produces a crisper crust. But, it has almost no effect on the temperature stability or thermal recovery of the oven, and the stone itself cycles in temperature. What about the last potential advantage- uniformity? Does the stone act as a "heat spreader", evening out the large thermal gradients from the electric elements, like a quality frying pan evens out a gas flame? The answer is yes, but also illustrates the limits of adding thermal mass... In the case of an oven baking stone, I measured the temperature at various points along the surface using a hand-held infra-red thermometer. And the stone performed well, varying by only +10F. Compared to hundreds of degrees on the aluminum baking sheet when the element turns on. So you can safely bake multiple rolls on the stone, and they will all brown simultaneously. A $2000 infrared camera could easily make this point. Or we could modify a cheap digital camera to boost it's infrared sensitivity. But, in the spirit of kitchen science, we can image the temperature gradient with just ten cents worth of parchment paper and the Maillard reaction. Take a sheet of parchment or quality rag paper. Using a paint roller, evenly spread out some thermally fragile organic goo- I find grape jelly or yogurt works well. Paint both sides of the paper so it won't curl, and let dry. Then carefully place above a hot surface (and keep a fire extinguisher near by- paper can burn, and its your responsibility alone to try this safely!) To test this method I place the sheet above a gas burner for one minute. Note how well the flame pattern is replicated. Then, after 10 minutes I turned off the burner, and imaged the residual heat store in the cast iron trivets.
Turns out there is not much to visualize in the electric oven - the uniformity of the stone is too high. But the situation is different in another "oven" where people often add thermal mass- the grill or smoker. This three burner gas grill is reliable, but annoyingly uneven. Triangular metal "roofs" shield each gas element to prevent grease flare-ups. As a result, hot gasses are concentrated in a few hot spots between the shields. And, the grill leaks air in from all directions to feed the flame, so the temperature can vary by 100F from top to middle to grating. After a while, you adapt yourself to the grill, and learn one spot is best for hamburgers, and another spot best for the thin edge of a fish tail.
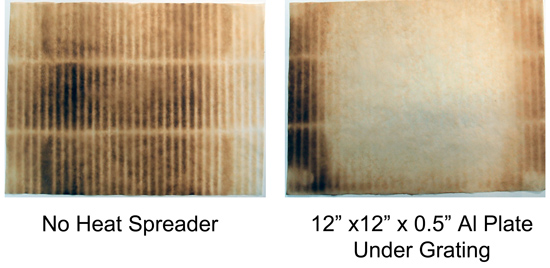
I compared the empty grill to the grill where I placed a thick aluminum plate directly above the flame shields. The darker spots indicate high temperature regions- note how effectively the metal plate averages out the hot spots (the clear middle is 150F cooler than the black areas). If a heat spreader works on a gas grill, you know how effective a baking stone would be in the calmer environment of an electric kitchen oven. This is also why I prefer to pan grill hamburgers and fish outdoors- amazing crust and easier to control.
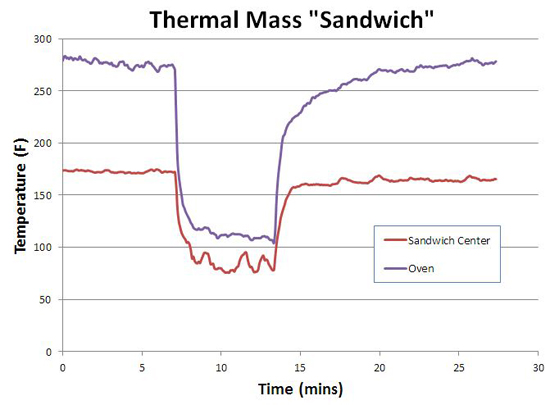
But while we have the gas grill set up, let's test an aggressive implementation of thermal mass. Some home bakers line the sides and bottom of their oven with stones, to add thermal mass and to replicate an all-stone hearth. Many barbecuers add steel plates to their smoker to reduce thermal variations. And these strategies, work, sort of. I constructed an oven-within-an-oven using two 12"x12"x0.5" aluminum plates stacked in a 1 3/4" high sandwich. This sandwich was placed on the grill between two hot gas elements set to medium, simulating indirect cooking in a smoker. One thermometer was in the center of the sandwich air gap, the other a few inches above the sandwich (approximately in the middle of the gas grill). It took almost 45 minutes to reach equilibrium. Note the temperature ABOVE the sandwich is higher than IN the sandwich. Hot air rises and is captured in the lid top, while cooler air supplying the flames with oxygen flows past the aluminum. Another reason to distrust the simple dial thermometer placed high above the cooking level.....
Then I opened the grill lid for five minutes. As you can see, the oven's temperature dropped immediately. As did the temperature in the middle of the sandwich, despite the warmth of two hot plates hovering less than an inch away!
After closing the lid, the sandwich recovered in temperature faster than the grill, so in this respect aggressive thermal mass offers a slight advantage. But, once again, this experiment illustrates the physics of heat transfer dominates over wishful thinking. Air is a very poor thermal conductor2, and once the grill is opened and outside air flows into the sandwich, air's poor conductivity cannot transfer heat from the plates to the air fast enough to prevent cooling in the sandwich. You can place your hand 1/4" away from a hot cast-iron handle with no pain-- touch the handle, and expect third degree burns. In smokers, air currents are a necessary fact of life, and these currents undermine the value of greater thermal mass. When cold air currents moves into the smoker past the meat, compensating hot air from a hot-rock moderator simply can't flow back to the meat fast enough- too much thermal resistance from the intervening air. This is true whether the thermal mass is a rock placed to the side of the meat, or from the thick ceramic walls in a komodo-style cooker. In fact, an active feedback system (like a PID controller adjusting the gas burners or coal air supply) kicks in more heat, on demand, than leaked in by thermal mass- beating a rock hand's down. An "ideal" cooker would be well insulated, rely on active feedback to keep the fuel source regulated despite external challenges, and place all its thermal mass in a heat exchanger between the heat source and the smoking chamber... So what have we learned?
|
|
--------------------------------------------------------------------------------------------------------
1 There is a slight decrease in oven temperature which does not recover when the stone finally warms up. This decrease comes from the stone physically shifting airflow in the oven towards the oven temperature sensor. The oven now thinks it is at a higher temperature, so decreases heating in response. 2 Most people think the glass in fiberglass insulation is the barrier to heat. But the thermal conductivity of glass is 40 times higher than still air. Primarily as a result of its higher density. Fiberglass insulation achieves its high insulative values by trapping air within a dense maze of fibers, preventing air currents that would otherwise transport heat directly. Air pockets are a very effective insulator, because there aren't many air atoms to carry the heat, and heat can only diffuse. Imagine putting out a fire with a three dimensional bucket brigade- the more volunteers to carry water from hand to hand (e.g. diffusion with more atoms), the faster the fire is extinguished. Carry water directly as a stream in a hose, and bucket brigades are a thing of the past. In a vacuum (i.e. no atoms), heat flow is very small, limited by thermal radiation. Which is why we keep our coffee hot in vacuum bottles instead of in fiberglass flasks. Recently, a winter storm knocked out our electricity for almost a week, and the temperature inside our house dropped to 43F. When the power was restored, the hot air furnace kicked in and within a half hour the AIR temperature was up to 68F, and we took off our jackets. But when we sat down on the couch, it was still freezing cold. And remained so for hours. Once again demonstrating- more tangibly than desired, how poor air is as a conductor, or as a reservoir of heat. |
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |