| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| solar cartesian diver |
|
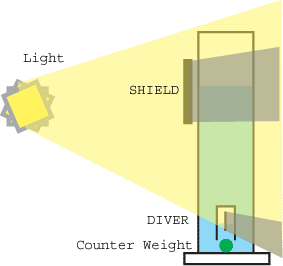
Every physics lab has a Cartesian Diver on the shelf to demonstrate the Ideal Gas Law and Archimedes Principle. The Cartesian Diver is assembled from an inverted glass cup, attached to a counterweight. Dropped into a tall container of liquid, the glass cup traps air within the diver. By adjusting the mass of the counterweight, so that the average density of the weight, glass and trapped air matches that of the surrounding liquid, the diver is made neutrally buoyant. Then, if the pressure outside the container changes (e.g. rising barometric pressure, or by sealing the top of the container with a rubber sheet and pushing on the sheet), the pressure on the liquid follows in lock-step, and is communicated through the liquid to the air within the diver. So, if the air outside the container increases in pressure, then the pressure within the diver increases. Push on air, and it compresses and increases in pressure. It will keep compressing until the pressure within the diver is the same as the pressure in the liquid just outside the diver. Water replaces the space previously occupied by the uncompressed air, making the average density of the diver higher than the surrounding liquid. The diver then sinks to the bottom of the container. Conversely, reducing the air pressure above the liquid causes the diver to rise. By pushing and pulling on the rubber sheet, the diver bobs down and up the height of the container. The Solar Cartesian Diver is sealed off from outside air pressure changes, and relies on temperature changes within the diver to create pressure changes. A thin black metal plate is mounted inside the diver. Light, from the sun or a lamp, goes through the liquid and clear diver wall, until it is absorbed on the plate. Sunlight can easily raise the internal temperature of the diver by 25 to 50 C. This resulting temperature rise increases pressure in the diver proportionately (by the Ideal Gas Law), forcing some of the water out of the diver. More air volume means a lower density, and once the density drops below that of the surrounding liquid, the diver rises. Eventually, the diver bobs to the surface, where it is hidden by a light shield. The diver cools, the air inside the diver contracts in volume, and it once again sinks to the bottom. And so the cycle repeats. Silently, smoothly, hypnotically. In practice, mineral oil is a convenient liquid. Mineral oil absorbs 10 times less solar energy than water, preventing thermal gradients and temperature increases in the liquid which might cause the diver to bob uselessly at the surface. Also, while a glass diver is practical in a laboratory environment, it cannot be shipped as a toy since the air will leak out of the diver when the package is inverted. Thus, a manufacturable device consists of a sealed clear plastic bag enclosing a black absorbing sheet. One open issue with this design is longevity- if the plastic absorbs oil it will increase in density, or if air diffuses out from the bag, it will decrease in volume. Careful selection of the diver plastic is critical. Finally, if the diver is designed for solar operation, the side of the diver facing the light is preferably a flat window. As opposed to a round tube. Otherwise, total internal reflection will prevent light from entering the diver except from directly perpendicular to the tank. If you are interested in constructing a Solar Cartesian Diver, the simulation will give you a feel for the challenge. I have never successfully prototyped a diver using solar energy and an unsealed column- however, with a strong (50 watt halogen), broad area light, it is possible for the diver to run continuously in an open column.
To see a movie of an actual Solar Diver in action (900 Kbytes, MP4 format), click HERE. To build a conventional Cartesian Diver from a soda bottle, try this SITE.
|
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |