| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| baked boiled or steamed |
|
July 2014
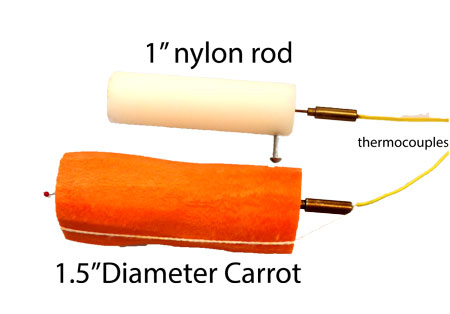
Planes, trains and automobiles. Which is the fastest way to get between two points? The most enjoyable? The cheapest? Of course, the answer is a conclusive "it depends". A plane is faster from NY to LA, but a train is quicker from NY to Philly. A car is more flexible and private than a plane (and you don't have to take your shoes off to "board" the aisle seat). But is flown by a trained professional, so you can sleep away the miles. With food, baking, boiling or steaming offers a similar array of trade-offs. Clearly, each method develops its own unique flavor profile. And sometimes, longer cooking times permits secondary chemical processes (like the conversion of collagen to gelatin in low and slow BBQ), to work its magic. But here we are mainly concerned with speed- is steaming the fastest method, and if so, why? And is it is *really* faster than boiling water? When steam condenses on a cold surface (like partially cooked food) it releases an extra packet of energy called the "latent heat of condensation". Which is much larger than the energy contained in the motion of the individual steam molecules (i.e. their temperature). The reason can be traced back to the affinity of water molecules to stick to each other tightly- which explains why they so easily form resilient, balloon-like droplets. By analogy, think of the steam molecules as tiny magnets. When they condense on a surface, they "click" together into a cohesive liquid, and that sound represents the release of latent energy from condensation. The latent heat of condensation is huge- around 2260 J/gm of water. Which means one gram of steam deposits 2260J of energy into a surface on condensation. The energy from thermal motion is much smaller. A gram of steam has an average heat capacity of around 1.8 J/gm-K. So at steam temperatures it contains around 1.8 J*(100C+273C)= 670 J, or three times less thermal energy than latent heat. A gram of dry air has a heat capacity of only 1 J/gm-K, or six times less than the latent heat. Which is enough motivation to compare steam to hot air baking. We tested two classes of samples. The first was a simple plastic cylinder. Since the nylon rod is dense and water impermeable, evaporation or absorption of water vapor is eliminated. Thus the plastic cylinder only responds to temperature and latent heat flow. The second sample was a carrot- which can cool by evaporation or absorb water. The carrot is a stand-in for all types of food:
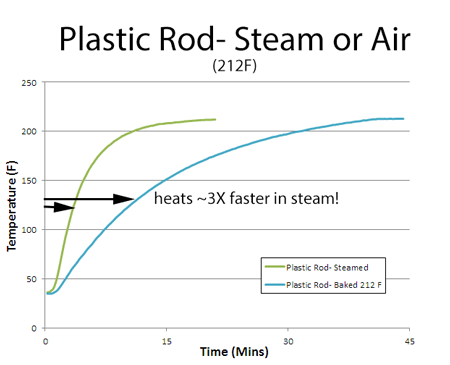
Before placing either sample in the oven, they were soaked in an ice bath for an hour to eliminate any thermal gradients between the surface and interior, and to start the cooking process at the same initial conditions. "Cooking" the plastic rod in steam or in a 212F oven (PID controlled for accuracy) was revealing. Steam cooks around 3x faster than air of the same temperature! Of course, it is easy to bake in a faster oven with hotter air (just like a plane goes much faster than a car), while steam is limited to 212F. But steam would still be 2x faster than a 400F oven. So the latent heat of condensation is a powerful cooking method:
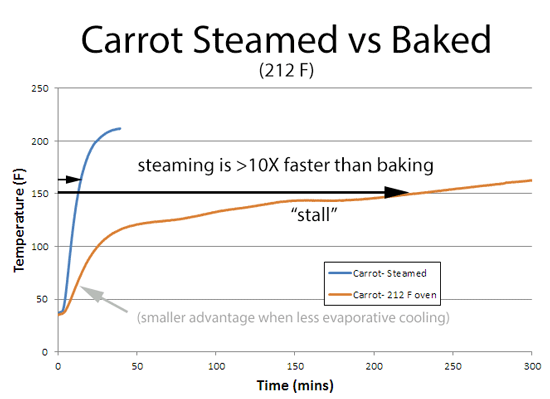
The advantages of steam are magnified with the carrot. Without steam, the carrot cools by evaporation into the dry oven air, and even "stalls" at one point. This dynamic cooling effect dramatically extends the cooking time from minutes to hours. But in a steam oven (like a sauna) evaporation is suppressed by the humid air, which is already saturated with moisture.
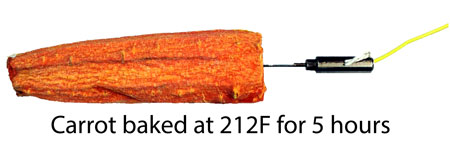
After 5 hours in a low oven, the carrot is well on its way to becoming vegetable jerky.
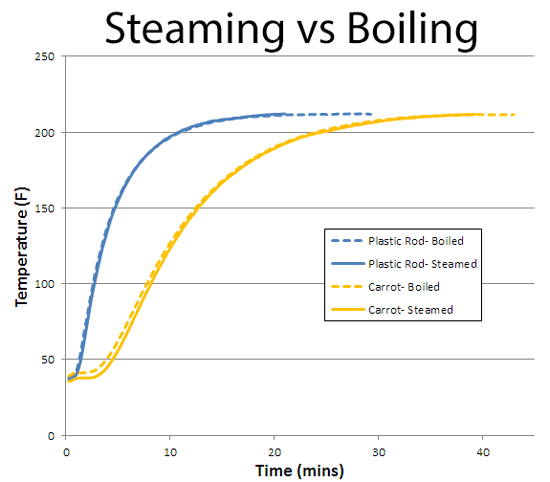
Sometimes, hot air vegetable baking is desirable- it concentrates flavors, and in a 400F oven layers in caramel flavors from the Maillard reaction. But in terms of speed, steam rules. How about steaming vs boiling? Many, many sources on the internet claim the same latent heat effect gives steam the edge. Even though water is much denser than steam. Others say boiling wins due to a higher degree of physical contact. But claims are not evidence, and the experiment only takes a few hours:
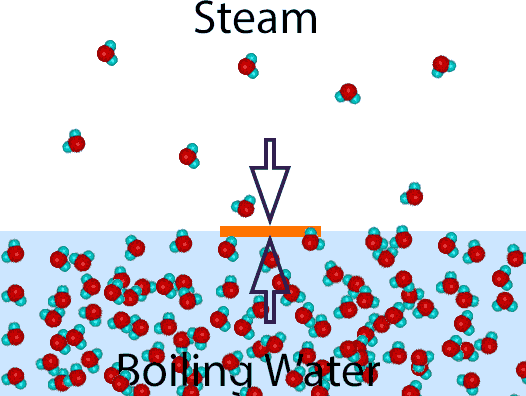
Amazingly, boiling water or steam cooks at EXACTLY THE SAME RATE! This can't be an accident, and it isn't. The latent heat of condensation may be huge, but the density of water is 600x higher than steam. So perhaps they cancel each other out? In fact, they cancel exactly, as this argument indicates: Imagine there is a thin sliver of carrot right at the surface of a boiling pot of water. The steam and the boiling water are both at 212F, so no heat passes through the carrot sliver in either direction. Which means any latent and thermal heat deposited by impinging steam, is exactly balanced by any heat conducted out of the boiling water. Thus the carrot cooks at the same rate- in boiling water or in steam1.
Given a speed a tie, this leaves us with the question: which method is better-- boiling or steaming? Well, it may take less energy to simmer a full pot of water than pay the latent heat of evaporation penalty to create steam. Unless you steam up a much smaller quantity of water into a sealed container, then steaming is faster. Vitamins are a mixed bag- some degrade faster in steam, some faster in water. I like the texture of steamed veggies, but others prefer boiled. So no clear winner. In summary-
|
|
--------------------------------------------------------------------------------------------------------. 1 This analysis is correct as long as its assumptions remain valid; in particular, that the steaming and boiling water are able to maintain 212F during the entire heating process. However, if a large and very cold object is cooked, it will cool off the air or water, and consequently will heat more slowly. For example, if a large cold object is placed in a small pot of boiling water, the water will cool down and boiling may take a bit longer than the steam to cook. Conversely, air (and steam) are poor thermal conductors. A large cold object in steam, especially where the steam flow is not turbulent or vigorous, will cool its immediate environment and in this case, steam will cook slightly more slowly than boiling. This difference is more pronounced if the object is an excellent thermal conductor, like aluminum or copper. A good thermal conductor will rapidly cool off its immediate surroundings before supplemental heat can arrive, exaggerating the slight rate differences. In practice, large pots of steam or boiling water will cook food at the identical rate, though may differ in either direction for some high thermal conductivity objects. Steam is sometime more effective than water when cooking a large number of small objects (e.g. potatoes or eggs). The steam is better at penetrating between the gaps and remaining hot, while the interstitial "boiling" water is often stagnant and cools off.
|
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |