| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| waiting for "Q" |
|
July 2011 One of the most frustrating stages of backyard barbecuing is the wait. Its not so much the tedium- when you get bitten by the BBQ bug, you sign up for ten, or twelve, or sixteen hours of tending fire before reaching pig heaven. It's not so much the fear- though cooks often write in a panic to blogs like AmazingRibs.com, pleading for help while the in-laws are impatiently waiting for dinner. No, its watching the meat thermometer stall midway through smoking- taunting you with its apparent disinterest, mocking your mastery of fire. Most cuts of meat tenderize only after the collagen begins to "melt" and the fibers begin to separate, around 170-190 F. Yet the thermometer dawdles at 155F all afternoon. What gives? Well, there are as many theories explaining the "stall" as there are hardened views on the Zen of barbecue. Some claim its moisture leaving the meat. Others claim the stall reflects the slow rendering of fat. Still others believe meat proteins are 'denaturing" at 150F, tenderizing and breaking down. A protein "latent heat". Or perhaps the wait is one last act of penance, to mollify the pig gods. Anyway, I performed a couple of tests, and believe the answer is pretty clear. As a baseline, the stall is easily observed in this recording from a digital thermometer inserted into the middle of a small brisket coated with a salt/sugar rub and injected with beef broth. The electric smoker was set at 225F, and a water filled drip pan was placed on a shelf below the meat: Note how the internal temperature stalls at 153F for almost six hours, then rises much more rapidly. Brisket can be a relatively dry cut of meat, and if you let it climb above 190F for very long, it ends up tasting "gray" and mushy. So don't get lulled into a sense of complacency by the steady thermometer reading. Walk away from the smoker at the wrong moment, and you might as well walk away from dinner. To separate the various competing theories, I performed the following test. First, I took a lump of pure beef fat from the fridge, inserted a tethered thermometer, and placed it into an unheated smoker. This is to test the "fat rendering" theory. I also soaked a large cellulose sponge in water, shook it out, inserted the thermometer and placed it on the same rack in the smoker. Then started cooking, setting the smoker to 225F. After a couple of hours, our faux briskets are coming along nicely:
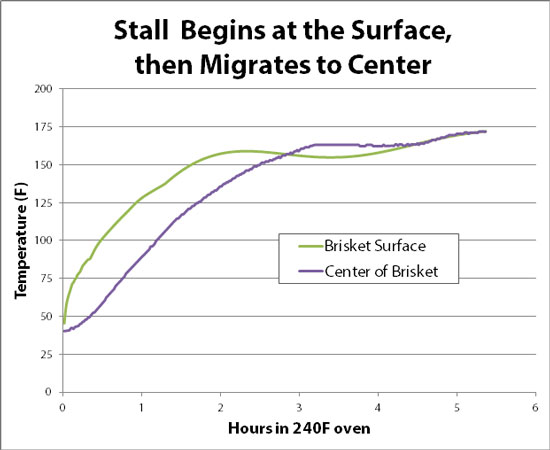
And here is the temperature plot: Note how the fat hunk continued to rise in temperature over time, while the sponge1 stalled- just like the brisket! Since there was a deep, glistening pool of rendered fat in the smoker, that particular hypothesis is busted (more detail here). And, we achieved "the stall" without the complexities of protein depolymerization- apparently its a physical, rather than chemical, effect2 (for that matter, even flour dough will "stall"). The most plausible explanation is a phenomena called "porous bed free expansion cooling", which is nearly as big a mouthful as a whole pulled hog. But the concept is actually quite simple. We are all familiar with evaporative cooling- soak a bandana in water on a hot day, and it will cool your forehead. Microscopically, what is happening is the faster moving water molecules in the bandana have the most energy, so most easily "freely evaporate" into the air by breaking through the water's surface tension (curiously, the hotter the water the faster the evaporation, because there are more hot molecules). Since the fastest water molecules escape the bandana, what is left behind are the slower molecules. That is, the colder molecules are left behind, which is why you feel refreshed. Evaporative cooling continues until the bandana dries out- just like the sponge dried out after five hours3 and quickly warmed to match the smoker's internal temperature. We can watch the cooling effect work its way from the surface to the interior, by monitoring not just the central temperature but also a mm below the surface. The exterior begins stalling an hour BEFORE the interior. And, it surface STOPs cooling an hour before the center, as the flow of juices wanes. In otherwords, meat stalls from the evaporating surface inward to the cooler center:
Conversely, fat doesn't evaporate, it simply melts. And contains too little latent heat of melting to delay cooking. So it can't be the origin of the stall. "Free" evaporation means evaporation into still, unmoving air. The alternative is "forced evaporation", enhanced by the cooling effect of moving air (as from a fan in a convection oven). The reason moving air cools more effectively is also simple- when a hot water molecule leaves the bandana, it might knock into an air molecule and careen back into the bandana. A humid aura surrounds any moist surface in stagnant air, reducing the benefits of evaporation. By stripping away the humid aura, moving air increases the probability that the evaporated water molecule stays evaporated. (Electric smokers are pretty well sealed, so evaporation is closer to "free" than "forced". Big stick-burning offset smokers are closer to "forced", and often have trouble building up a strong smoke profile before the meat's surface dries out.) A "porous bed" is simply a solid reservoir of water. For example, a sponge is porous and can hold water without spilling. So are the walls of a terra-cotta pot, or garden soil. Or even meat. The key attribute of a porous bed is control. It slowly meters out water from the interior to the exterior at a steady rate, where it can evaporate and cool. Basically, the tiny pores and cracks within the bed create a great deal of viscous friction, limiting the outflow of moisture. Think of it as a water battery, with a resistor limiting the current. Porous-bed-expansion-cooling is widely deployed as a low tech cooling technique. For example, moist nested terra-cotta jars are the basis of a simple but effective refrigerator. In low humidity areas (like the desert), swamp coolers substitute for air conditioners. And green roofs help keep buildings cool. So, I conclude the barbecue stall is a simple consequence of evaporative cooling by the meat's own moisture, slowly released over hours from within it's pores and cells. As the temperature rises, the evaporation rate increases - until the cooling effect balances the heat input. Then it stalls, until the last drop of moisture is gone (kind of like your car engine- which can drive at 60 mph until the last ounce of gas is burned and you stall on the side of the road). This supply of moisture is limited, and many complex effects present in meat, are missing in a sponge (such as collagen liquefaction and de-polymerization), which squeeze out moisture even faster than evaporation4. The stall is rapid in dry environments, prolonged in humid. Once the meat's ability to supply moisture peaks, it gradually starts to over-heat. Leaving fats and other oils to supply moistness. I suppose you could speed up low-and-slow barbecuing by introducing a fan and a dehumidifier into the smoker. But I think I'll stick with tradition5.
|
|
--------------------------------------------------------------------------------------------------------
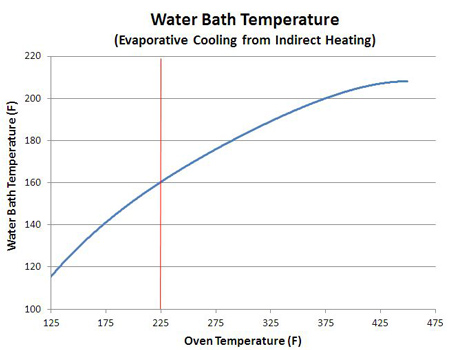
1An astute reader might notice the sponge's temperature not only stalled, but actually declined a bit over time. The reason is quite interesting. The sponge was initially soaked in water, and that water completely filled many of the surface pores, reducing the surface area of the sponge. Think of the beach at high tide- initially the water line is smooth, but as the ocean recedes, more and more rocks and nooks and crannies are revealed, increasing the surface area. As the water evaporates, its like the tide going out. Additional pores are uncovered, leading to greater evaporation and higher cooling rates. Until the last drop evaporates. Finally bone dry, the sponge quickly heats up. A smooth piece of meat has much less surface area than a sponge of the same size, so the sponge releases moisture faster than the meat, and thus evaporatively cools to a lower stall temperature (e.g. compare the brisket curve to the sponge curve above). Additionally, as the sponge slowly dries, it becomes lighter, meaning there is less mass to cool. Given the same evaporation rate but progressively less mass, the temperature of the remaining liquid will cool faster. Small things cool faster than large things. Which is why the effect is so visible- the sponge loses 90% of its weight, while the meat perhaps 20%. Other factors affecting the stall temperature: In many cases, the "surface to volume" ratio controls how fast food cooks. A flat pizza pie cooks faster than an oblong loaf of bread. Match-stick french fries crisp up faster than thick steak fries. A large piece of meat contains a greater proportion of stored water, relative to its surface area, than a small piece of meat. So it can supply more evaporating liquid, per inch of surface area, than it's smaller brethren. And thus eventually cool to a somewhat lower stall point. Evaporative cooling does not require a porous bed, of course. Even a small cup of water in the 250F smoker will evaporatively cool, as you can see by the beginning of a stall in this graph at 50 minutes. Repeating this stall experiment at multiple oven temperatures yields this curve:
While the curve's shape depends in detail on the type and configuration of each oven, it's clear why meat often stalls around 160F when cooked low and slow - like a simple water bath, evaporative cooling lowers the meat's temperature to 160F when cooked at 225F. The stall temperature also depends on the air velocity. A kitchen oven or a well-insulated electric smoker barely vents oven air to the exterior. Basically, there is little air motion inside these cookers. A pellet smoker, on the other hand, often requires a fan that actively pushes air over the burning pellets and into the cooker. By measuring air velocity near the vents, calculating the vent area and the volume of the cooker, its possible to calculate the number of air exchanges in an hour. A home oven might change out its own volume a few times an hour- a pellet cooker over 100 times an hour. The faster air moves over a wet surface, the greater the cooling effect and the lower the stall temperature, as may be seen in this water bath stall data from a kitchen oven (blue) compared to a pellet smoker (red). As much as 25F lower in the wind- that's why windy days feel like cooler days. This lower temperature slows down cooking rates, while the faster evaporation rates more rapidly desiccates meat so it can break through the stall- depending on its size and shape and type of meat, more air flow can speed up or retard cooking.
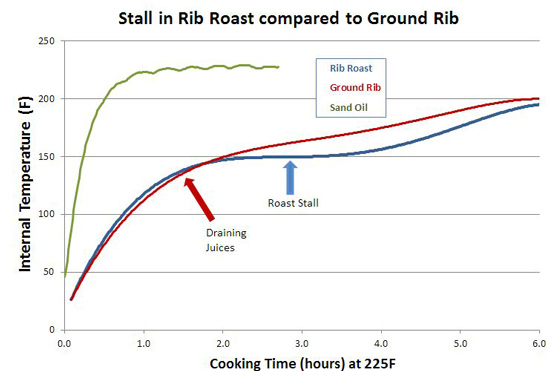
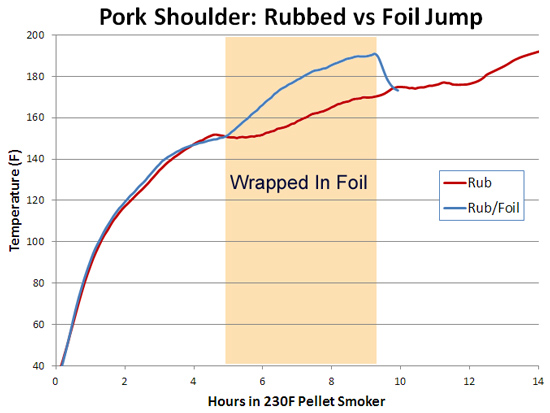
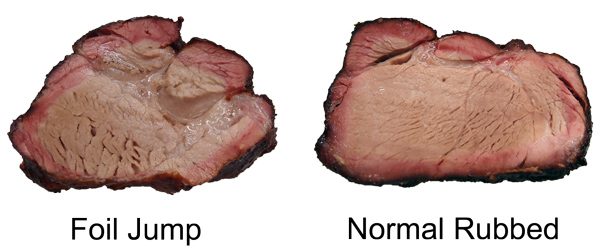
You can also see the effect of air currents in this experiment, testing whether spritzing meat during smoking slows down the cooking time. Here I sprayed a pork tenderloin in a kitchen oven at 250F, and one in a pellet smoker at 230F. Twice an hour, but careful to only open the oven for 15 seconds at a time. The "control" were tenderloins of the same size and shape, unsprayed. While you can detect gentle ripples immediately after spraying, the cooling is modest and recovers quickly. 2Around 25% of the total protein weight in most animals is collagen, primarily in the skin, tendons and bones. In the cuts of meat typically consumed, on average collagen makes up less than 2% of the weight, and sometimes as low as .2%- much less than the fat or water content. And not all of the collagen "melts" during cooking. Even is we assume a latent heat as high as in water, there just isn't enough collagen available to explain a stall at any cooking temperature. We can also rule out a collagen melting phase transition by two other experiments. If you wrap meat in foil so evaporative cooling is blocked and measure the meat's temperature rise, no stall is observed (Nathan Myhrvold, among others over the decades, has made this same point). But the melting phase transition should be unaffected by the foil. Also, if you cook meat in a hot oven, the stall disappears. This again rules out a phase transition- think about a pot of ice in an cooker- it will stall at 32F whether in a mild or roaring oven, until the last ice cube melts. Just the length of the stall will vary. 3I attached a small string to the sponge, and could weigh it during smoking. 4As anyone who's ever cooked a hamburger knows, juices start to flow profusely from the patty around medium rare (135 F). Its finely-ground meat texture offers endless channels for drainage- which I tested by comparing an 8 oz. well marbled rib roast slab to another 8 ozs of the same roast, passed through a food grinder. Note how the solid piece of meat stalls for two hours around 150F, while the ground meat (RED curve) simply continues to heat up. Actually, around 130F the digital thermometer began jumping around by + 5F degrees every few seconds as the meat juices spurt out, much faster than needed to evaporatively cool. Then it calmed down around 150F. By the time the burger reaches well-done (155F or, shockingly for some carnivores, above 155F), the meat is pretty well desiccated. It's still cooled slightly by evaporation, and cooks slower than sand. But the roast retains enough moisture to continue stalling for another hour and a half. The ground meat evaporatively cools until it dries out (around 150 F), and then it acts more like the sand cup. Which just about describes the taste of well done hamburgers. 5The role of humidity inside the smoker is also hotly debated. Many people add a water pan to "keep the meat moist". Others swear that constant wet mopping at low temperatures is the secret to great 'cue. Which may be true, except a number of contest winners have demonstrated that high, fast and dry leads to equally delicious results. There is no question extra humidity, whether via a water pan or wet mop, will slow down the cooking process. In low-and-slow this allows the 'cues interior to catch up with the surface temperature. But I doubt it makes a significant difference in the meat's eventual tenderness. The fact is, until the meat dries out enough to reach 190F or so, it won't develop the complex flavor profile indicative of great barbecue. On the other hand, I do believe even subtle differences in time and temperature and chemical gradients across the meat leads to equally subtle flavor contributions. Taste is a wonderfully nuanced sense, and these tiny differences can be perceived, and separate the good from the best. So there may be science behind the black magic. One can split the difference- smoke until it just reaches the "stall", then wrap in foil to essentially braise the meat in its own juices, and then unwrap near the end to crisp the bark. The initial stage incorporates enough smoke flavor that wrapping for the remainder of time isn't an issue. And, unless you continue to mop, the smoke ring is fully developed. But, wrapping does allow a more rapid temperature rise because the impermeable foil entirely blocks evaporative cooling. Just don't expect the temperature inside the meat to immediately hit 212F- air is a poor thermal conductor and it takes a lot of heat to raise the temperature of a big hunk of pork. Air is 1000 times less dense than meat- it simply contains too little energy to flash boil water in the foil. If you don't wrap tightly (and I mean water tight), the juices will stall well below 212F. But eventually it will heat up, and the extra humidity helps collagen break down into gelatin. Save the juices that collect in the bottom of the foil and reduce as a final glazing liquid. The juices contain most of the smoke flavor washed off the bark by the braising liquids. This graph shows the effect of both methods on two, 3 pound pork shoulders cooked in a 230F pellet smoker without a water tray. Both were initially rubbed with a salt/sugar/spice mixture. The foil-jump meat is tender at 10 hours, where as the normal rubbed pork took almost 14 hours to reach perfection. Note the temperature DECLINES after unwrapping- evaporative cooling back at work. The flavor profile is slightly different for these two methods, and you will have your preferences, but either approach will win awards.
And either interior will pass muster:
You can cook even faster by placing the wrapped meat in a much hotter oven - just don't let the meat's internal temperature climb above 190F during the braising step, or it will slip from tender to mushy. But 190-195F on the grill is fine- humidity makes all the difference.
|
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |