| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| waiting for the vodka to go |
|
August 2011 Baking a crispy, flaky pastry shell is something of an art. Pie fillings tend to weigh down the dough, resulting in a soggy base and overcooked rims. If we could somehow cook the dough faster, or knead a drier dough, the pie shell might be crisper. But how? A few years ago Cooks' Illustrated popularized a half century old recipe where vodka was added to the dough in lieu of water, claiming in their words:
To test this hypothesis, two flour spheres were created by adding water or vodka (80 proof) to 1/4 cups of all-purpose flour, and kneading for a mere 30 seconds. The spheres were the size of golf balls and weighed around 1.75 oz. No butter or fat was added, as we were simply testing the effect of vodka vs water. Thermocouples were inserted into the center of each sphere, and baked in a 300F convection oven until they attained an internal temperature of 300F- that is, until all the moisture was driven off and evaporative cooling ended. Baking, until crispy, took over two hours. Both spheres appeared identical, at least externally:
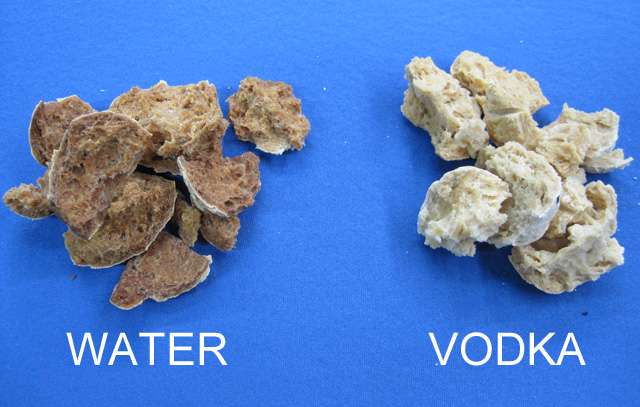
But after cracking open with a hammer, they couldn't be more different in appearance:
Under magnification, the water sphere is brown and filled with bubbles connected by strands typical of baked bread. On the other hand, the vodka sphere was lighter in color and flakier in appearance- like a pastry shell. How is this possible?
The key is gluten and miscibility. Flour contains a long-chain polymer protein called gluten. When lubricated with water, the gluten strands straighten out during kneading (rather like combing wet hair), forming a tough elastic microstructure of fibers and sheets which capture gases like a balloon. Water is weakly retained by the gluten structure (but not strongly chemically bonded), so will boil off when heated. Proteins, sugars and other chemicals are needed to promote browning in the Maillard reaction, which occurs even at room temperature but speeds up around 300F.1 By straightening out the gluten molecule, enough protein was exposed on the "water ball" to brown during the experiment. Conversely, the vodka side almost looks like pastry dough, where a fat, such as butter or lard, was kneaded into the flour. Fat (like oil) does not mix with water, so the pastry separates into distinctive, floating layers of moist flour lubricated by sheets of fat. Less exposed gluten protein is developed, which means less browning. Resulting in a lighter and flakier cooked dough. But we did not add any fat to these spheres, at least not directly. Never the less, flour, even after the oily wheat germ is removed, still contains a small percentage of fatty lipids. The vodka (which is 40% ethanol alcohol) can dissolve out these lipids (and a bit of the gliadin, weakening the gluten sheets), which then lubricate and separate the flour into layers, just as if we had introduced cold butter. It also helps break down the gluten strands, softening and tenderizing the dough. But dry flour does not readily absorb moisture. This is why you flour a counter-top to make sure wet pizza dough won't stick. In a pie crust, when you knead water into the butter/flour mixture to finalize its pliability, the water may bead up as it is dribbled in. If these beads do not soak into the flour, they create little hard grains of dried dough, almost like a coarse-ground corn meal. Very unpleasant, and all too common. Interestingly, vodka can play a role here as well. "Hard water", which contains dissolved minerals like calcium or magnesium, is very reluctant to break the flour/water surface tension barrier. This is because one species of mineral ions bonds to the flour, and their anchored charge prevents the oppositely signed mobile ions from wandering away. But, if you dribble soft water (that is water lacking minerals) into dough, the flour absorbs the moisture freely. No ions, so no electrostatic charge barrier. Vodka is even more effective than soft water- vodka is a solvent for the oils, helps break apart the proteins, and lowers the surface tension to flour2. And the result is astonishing: We compared all three liquids in this short movie. A bowl of flour was leveled with a knife, and three small depressions (3x5 mm) were embossed into the dough. "Hard" water is a weak solution of either baking soda or calcium chloride. "Soft" water is distilled water available at any supermarket. The vodka is unflavored and 40% by volume. Note while the vodka is absorbed in 15 seconds and the distilled water in a minute, it can take longer than 10 minutes for the hard water to penetrate the surface. Salt water or dilute vinegar takes longer than soft water, but faster than hard. pH also determines how fast water hydrates flour- see this article for details on the role of pH and salt. Now back to vodka and how it leaves the dough during baking. We can discern the movement of alcohol within the dough spheres by monitoring the internal temperature of each sphere during cooking. Like barbecued brisket, the internal temperature "stalls" for hours as the evaporating moisture cools off the heating dough. Note how vodka, with its lower boiling temperature, evaporates more rapidly than water and so more effectively cools the sphere. The long flaky microstructure also makes it easier for ethanol to move rapidly from the interior to the exterior, compared to the more tightly intertwined water sphere.
This more porous structure also explains why the vodka sphere stalls (1.5 to 2.5 hrs) at a lower temperature than the water sphere- it has a greater effective surface air so water cooling is more effective. Like a radiator with more fins. Finally, notice how the vodka sphere dries out a half hour earlier- after all, it contains 40% less water, with its much greater latent heat. Adding vodka to flour performs FOUR functions. First, on a volume basis it contains 40% less moisture than water. Less water develops less gluten, softening the dough, and enabling it to dry out faster. Secondly, the alcohol-extracted lipids modifies the kneaded structure, creating a flaky microstructure. Third, it allows moisture to uniformly penetrate the dry flour, eliminating grittiness and improving uniformity. And fourth, vodka helps break apart long gluten chains, improving tenderness. Plus it stalls like a barbecued brisket. What more can you ask for an afternoon's experiment in the kitchen?
|
|
--------------------------------------------------------------------------------------------------------
1We chose a baking temperature of 300F intentionally, as this is right on the cusp of the Maillard reaction, so tiny differences in protein composition are amplified by the browning reaction. At 400F both spheres would darken. You can see the effect of higher temperatures in our pizza dough experiment. 2 Commercially, surfactants and emulsifiers are added to dough to improve wetting and uniformity. Molecules like lecithin, cetyl alcohol and polysorbate. Easily available on line, if you are adventuresome, try this at home. 20% vodka is also effective, but 10% is similar to distilled water. Or, you can eliminate the water step entirely by adding moisture in the form of a water/fat emulsion. For example, with mayonnaise. Or, rush out and try the wonderfully rich and flaky cream cheese crust perfected by Rose Levy Beranbaum. Not only is the water intimately bound to fat, but commercial cream cheeses almost always include a chemical emulsifier in the ingredient list.
|
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |