| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| slip slidin away |
|
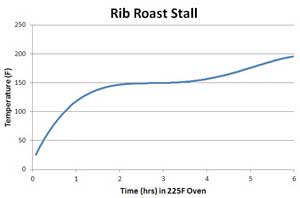
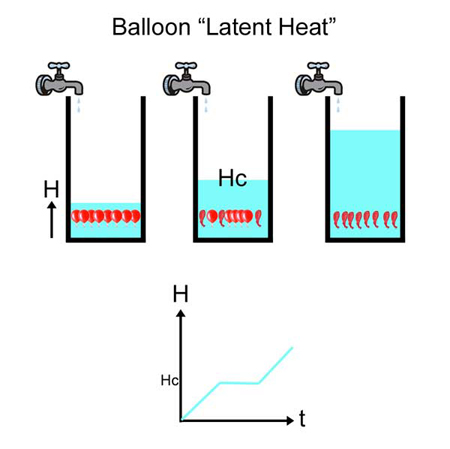
Oct. 2012 Juicy Latent heat lipid phase transition? What does that have to do with delicious food, perfectly cooked and dripping with flavor? Actually, not too much. But this is a case where a little knowledge, vaguely recalled from high school science class, is a dangerous thing. And an object lesson reminding us that simple explanations, based on mutual resemblance, are simply not always right. Our story has two parts. In the first half, the mystery of the "stall" is revealed- a period during meat cooking where the temperature stubbornly refuses to rise, plateauing for minutes or hours, still tens of degrees away from done. In the second half of the story, we learn why fat is a slippery word. The origin of the stall is clearly a result of evaporative cooling- see this article for a more thorough explanation of how this phase change from liquid to gas prevents the meat from warming-- but other hypothesis have been proposed. Most notably a melting phase transition from solid to liquid. A phase transition is the conversion of one form of matter into another, often abruptly so. For example, ice melting into water changes the phase from solid to liquid. Or, dropping hot iron (plus a little carbon) into cold water converts a soft metal into hard steel. Converting one phase to another generally absorbs (or liberates) heat to rearrange its atomic structure. This stored energy is called a "latent heat". A simple analogy for latent heat is a balloon in a bucket of water. Imagine the balloon can be in one of two "phases". The first phase is "inflated" and the second phase is "deflated", but this is a special kind of balloon that rapidly collapses when placed under a critical amount of pressure. Now imagine adding water to a bucket containing balloons (tied to the bottom, of course). The water level (a surrogate for temperature) rises when the tap is turned on (e.g. placed in a oven adding heat). When the water level is at the critical height Hc corresponding to the critical pressure, one of the balloons collapses. Which opens up a little space, slightly dropping the water level. But since water continues to flow in, the level rises again to Hc, triggering another balloon collapse. This process continues until the last balloon collapses, but until then, the water level is basically stagnant. Once all the balloons have collapsed the phase is now fully "deflated", and the water level rises continuously. Of course, the process reverses itself if the tap is turned off and the water drains out (e.g. by analogy, the bucket is "cooled").
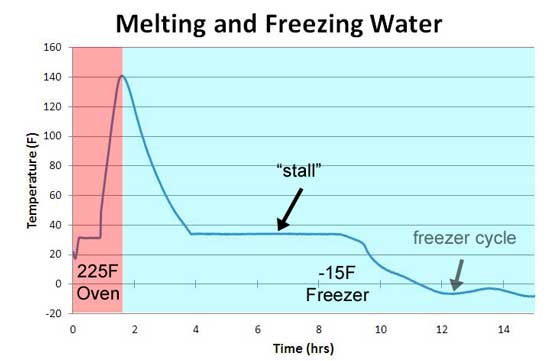
Now meat contains collagen- a tough protein molecule which surrounds and locks together muscle fibers. At high temperatures- above 160F or so and in the presence of water, collagen very slowly converts to gelatin. Gelatin is a smooth, unctuous gel which lubricates the muscle fibers instead of locking them together- converting a tough matrix into a tender and easily chewed delight. What if this conversion of collagen to gelatin - a phase change- absorbed energy from the oven, preventing the meat from rising in temperature? In high school science, you may have seen or even measured the phase transition in water. Below is an experiment I performed on a test tube of ice with a thermocouple mounted dead-center1. The ice rod was then placed in a 225F oven. Note how it stalls at 32 F as the ice transitions from solid to liquid. In this transition the ice is absorbing heat from the oven to break the bonds maintaining its crystalline order, just as the collapsing balloons "absorbed" water volume. When the water reached 140F I removed the tube from the oven and placed back in the freezer. The water temperature dropped smoothly, until once again it reached 32F- stalling while it released heat as the water molecules locked back into icy alignment. So indeed, a phase-change looks a bit like the stall experienced during cooking.
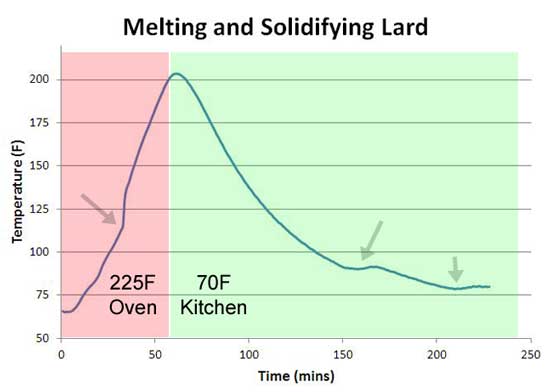
But there are two problems with this hypothesis. First, while it's true a cow is nearly 30% collagen by weight, almost all of that protein is locked up in the skin, tendons and bones. Muscle meat contains just a percent or two of collagen. Somewhat like the nails in a building-- just a little bit goes a long way towards holding the frame together. Unfortunately for the the hypothesis, 1% of any protein is simply too little mass to explain a three hour stall2. So collagen is ruled out, but perhaps melting fat is just the phase transition we need. A steak might contain 30% or more fat, along side it's 1% of collagen. Meat fat consists of "lipids" like stearic acid, which melts at 160F, palmitic acid which melts at 145F, lauric acid at 110F and others. Sometimes individually, sometimes bound to glycerin in groups of three as a triglyceride. The exact mixture depending on the breed, diet and age. But with a blended chemical make-up, a potential crisp flat phase transition tends to average out. We can see this averaging in a test on lard (purified pork fat) under conditions similar to the ice experiment above. While a few bumps in the melt/freezing curve are visible, they are no longer very distinct. Averaging is even a stronger effect in real meat, where different sections cook at different temperatures, the lipids are in various states of unraveling, etc. And did I mention there are 19 different forms of collagen? So a phase transition, in a collection of large molecular compounds embedded within a muscle matrix, is an unlikely explanation for the stall. Water, a pure molecule even in meat, is a more likely candidate.
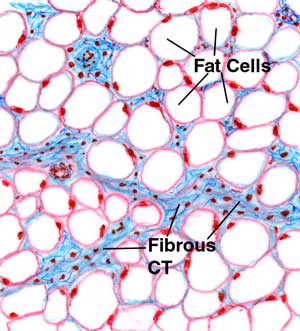
The second problem with the hypothesis is fat is more than the sum of its parts. Which leads to the final half of this story. If fats starts melting around 110F, why is there any fat left to drip down my cheek from a pulled pork sandwich, which was smoked until it reached >185F? It turns out what we think of as smooth white fat ("adipose tissue") are really cells containing fat molecules stored away for later use as fuel. Not only are there cell walls, acting like bladders retaining the fatty lipids, but there are collagen and capillaries and some water and other stuff too numerous and unfamiliar to list. When you gently heat fat in an oven, some of the molecules melt, burst their cell walls, and drip out. But other molecules melt and are contained in the cell bladders. Or don't melt at all, because the melting is suppressed by its chemical environment
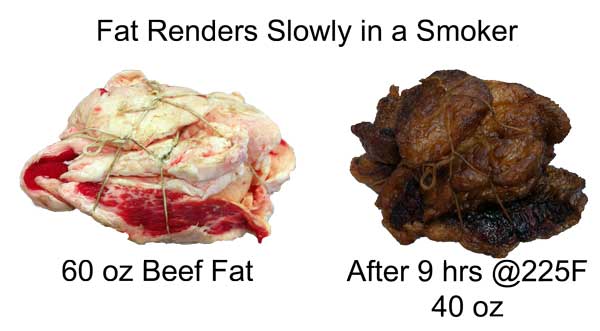
Fat cells-stained for visibility The slow rendering of fat in an oven is dramatic- this 4 lb. lump of beef fat trimmings reached 185F after 9 hrs in a smoker- yet 2/3rds of it is unmelted. Good thing too- otherwise eating a beef brisket sandwich would be like gnawing at a cellulose sponge.
Phase changes are the stuff of life. But when you learn about this complex phenomena in science class, most books simplify the explanation by using purified examples drawn from the laboratory. The real world is more complex, and in this case, much more interesting and tasty.
|
|
-------------------------------------------------------------------------------------------------------- 1Measuring phase transitions can be difficult. In a large sample of material, the inner and outer temperature of the sample may vary radially due to thermal gradients from the oven to the interior of the sample. So what might be a sharp transition visible at one temperature, is actually a collection of small precise transitions averaged over many temperatures. To avoid this problem, the test tube is only 10 mm in diameter and placed in 4" of fiberglass insulation, to allow the water time to achieve a uniform equilibrium. Thus explaining why it took hours to re-freeze. A home fridge is not a constant temperature bath- as the compressor cycles, the temperature rises and falls by a few degrees. Throw in the effect of impurities on melting temperature, super-saturation and other gremlins, and you can appreciate why precise measurements take an expert's hands and equipment. 2We can also rule out a collagen melting phase transition by two other experiments. If you wrap meat in foil so evaporative cooling is blocked and measure the meat's temperature rise, no stall is observed. But the phase transition should be unaffected by the foil (Nathan Myhrvold, among others over the decades, makes this same point). Also, if you cook meat in a hot oven, the stall disappears. This again rules out a sold phase transition- think about a pot of ice in an cooker- it will stall at 32F whether in a mild or roaring oven, until the last ice cube melts. Just the length of the stall will vary. |
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |