| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| chill out |
|
Oct. 2012 Among the legions of dark mysteries standing between your lips and the enjoyment of a fine bottle of wine is learning to serve the wine at just the right temperature. Unfortunately, the "proper" temperature is always colder than an invitingly warm dining room. So the wine must be chilled. If you don't own a wine cellar, chances are you plan on cooling down a recently purchased bottle- a bottle that procrastinated in a warm car on its long way home from the liquor store. There is never enough time to prepare dinner, so a fast cooling method is imperative. Now the most effective cooling method is direct contact with a cold liquid- plunge the bottle into a bucket of ice (or even better, salted ice which drops the temperature another 20F), and the wine cools in just a few minutes. Yet a bucket of salty water can be a mess. Sometime the label even slides off in the process- nothing like serving your guests an anonymous vintage. So you might be tempted to place the bottle in the freezer for a quick, dry cool-down. The problem is air is a much poorer thermal conductor than water- between 25 to 100 times worst. You don't need to be a scientist to realize if you place you hand in ice water it hurts immediately- but your fingers are only slightly discomforted poking around in the freezer. Is there anything we can do to speed up the cooling process in air? Two possibilities suggest themselves. First, you could place the bottle on a wet towel, so heat conducts from the wine, through the towel and then directly into the cold shelving. Or, you might wrap the bottle in a moist towel, hoping water evaporation will cool the wine (the freezer is drier than the warmest desert, so evaporation is potentially very effective). So let's try a few tests. The first is modeled on a suggestion made by Cook's Illustrated. I wrapped a 750 ml wine bottle in a moist towel1, and drilled a hole through the cork so I could insert a thermocouple into the dead-center of the bottle. This bottle was placed on a shelf in a freezer, next to a bottle with an identical thermocouple but lacking a moist towel. Note the bottle's orientation matters. A horizontal bottle will cool faster than a vertical bottle, because convection currents are stronger when the convection cells do not have to travel the long distance from the bottom of the bottle to the top of the neck. So all these experiments are with a horizontal orientation. Here are the results. Assuming a proper serving temperature around 50F, there is hardly any difference at all between the two bottles, but even over the course of an hour, the moist towel wrapped bottle took LONGER to cool than the dry bottle.
Why longer? Well, its true the moist towel potentially could improve thermal conduction to the shelf compared to air. But, the towel is not really a great thermal conductor- between the cotton, entrapped air bubbles and the added mass which must also be cooled down, it's more of an impediment than an accelerator. To see why, I froze three 1 L water bottles (it too painful to freeze another perfectly good bottle of wine, and in any case, the thinner plastic bottle eliminates the slower thermal path of a thick wine bottle glass wall). One bottle was plain, one tightly wrapped with a SINGLE dry paper towel, and one wrapped with a dry cloth towel: Again note the plain, unadulterated bottle cooled fastest3. Even a single paper towels traps enough air to be a pretty effective insulator, almost doubling the freezing time. I also placed the bottle on a folded wet paper towel, to increase conduction into the shelf. It cooled at exactly the same rate as the plain bottle- in other words, acted as if it were un-insulated and did not show evidence of improved direct thermal contact. Finally, I tested the idea that evaporative cooling might be effective. Again, I used three water bottles with identical thermocouples inserted dead-center, but in this case, I wrapped one of the two moist cloth bottles with saran to prevent evaporation:
And the results: The saran wrapped towel did cool slower than the exposed moist towel as a result of decreased evaporation and perhaps a bit of entrained insulating air. But the plain bottle cooled fastest. Again. It turns out it's very difficult to thermally "sink" the wine bottle to the freezer with a simple wet towel. The towel adds mass and insulation, which overwhelms the potential advantages of direct thermal conduction. But what if we enhanced the effect of evaporative cooling, by adding a fan to the freezer? Sort of an alcoholic "wind chill" factor. Certainly, if your clothes get wet while hiking on a brisk winter day, you can easily die from exposure- Hypothermia3 is insidious. Perhaps if we created a mini-blizzard in the freezer... Or a gentle breeze. I placed a small, 6" fan scavenged from a computer in the freezer next to a 1L water bottle swaddled in a moist cotton towel. The fan created a 4 mph air current in a nearly empty freezer, chilling the bottle (solid blue curve) almost twice as rapidly as a control (dotted blue curve, a moist towel wrapped bottle merely exposed to stagnant freezing-cold air). It chilled even faster than a plain water bottle, with or without air motion (compare solid blue to either pink curves, blue curves moist, pink curves dry for comparison4). As everyone knows, a wet towel and a box fan offers a cooling respite on a hot summer day.
Some home-freezer models do contain a small fan, gently teasing air into motion. But the velocities are low, and real-world freezers are stuffed to the gills, blocking air from circulating between last Christmas' ham and those frozen dinners no one likes to eat anymore. In other words, only in a commercial blast freezer does wrapping a wine bottle with a wet towel work. In a home freezer, it actually DELAYS cooling. So what should you do when in a rush to serve wine with dinner? If you only have ten minutes to spare (and shame on you for drinking a nice bottle of wine in a panic), a bath of ice water will get the job done in 15 minutes5. Direct thermal conduction is very effective. Prefer a drier method? Place the bottle in the freezer (-15F) and it will reach 50F in about a half hour, depending on its initial temperature. Have a bit more time on your hands, and don't want to risk freezing the wine by accident? Store in the fridge (34F) and wait about an hour and a half. And next time, plan ahead.
|
|
-------------------------------------------------------------------------------------------------------- 1The terry cloth dish towel weighed 60-70 gms. I tested both a moist towel (80 gms water) and a soaking wet towel (240 gms water), and neither cooled faster than a plain bottle. 2The Cook's explanation for how it works-- "Since cooling occurs when heat is transferred away from an item, the water in the towel—a much more efficient conductor of heat than air—will quickly freeze, dropping the temperature of the wine to 50 degrees in only 30 minutes", is suspect. True, water is a better conductor, but the moist towel still has to transfer heat to the freezer's air- which remains the primary impediment to cooling. Perhaps they placed the wine in a blast freezer exposing the moist towel to a brisk stream of -25F air. 3Hypothermia is a silent killer, but we can apply insights gleaned from our wine experiments to help avoid a bad ending to a very good hike. Assume, for a moment, you are walking across a snowy brook on a cold and windy January day, and break through the ice. Soaking your clothes and your pack with ice-cold water. Death could be lurking just a few minutes away. What should you do?
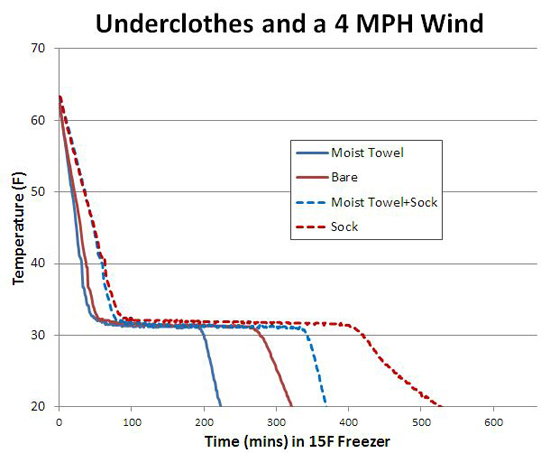
Even a thin layer of dry underclothes makes an enormous difference buffering your skin from wet clothes. Here I compared two 1 L water bottles as surrogate "calves". First, I slipped a lightweight "Dri-release" Wigwam cotton/polyester hiking sock over each bottle, wrapped one sock with a moist cotton towel, and then placed both bottles in the freezer (-15F) along side the 4 mph fan. Note the presence of the sock (about 2 mm thick after stretching) doubles the cooling time, even when covered by a moist towel. Remarkably, in the first hour the moist towel + sock is almost as well insulated as the dry sock alone (dotted lines). Because the human body generates heat, most survival guides advise placing your thick damp clothes over the thin dry underclothes- in a while, the moist cloth will dry out and you will be safe and warm.
Frostbite is a result of evaporation as much as the cold. If you wear gloves or mittens on a cold windy day (say 0F and 15 m.p.h. winds) your fingers can become frostbitten in a half hour. But, if you wear a thin pair of latex or nitrile disposable painting gloves under the mittens, you will stay toasty warm all day. Make sure to wear the rubber gloves under your regular winter gloves. If you wear them on top, your hand's moisture condenses inside the wool or polartec, which gets wet and stops functioning as insulation. But, if the rubber gloves are worn directly on your hands, the insulating fabric gloves stay dry and warm. It's the same reason why builders staple a moisture barrier on the INSIDE surface of a cold wall, facing the hot moist interior air. Otherwise, the insulation becomes soggy. The only downside is minor- your hands will be swimming in sweat after a few hours- but your glove insulation will be dry and effective. Vapor barriers are frequently a good idea in sleeping bags, but may become clammy, as this outdoor hiking club reports. To demonstrate the effect of wind and vapor impermeable gloves, we chose a skinless hot dog as a surrogate for a human finger. While there is no blood flow in the hot dog and thus the temperature drop is exaggerated, the relative cooling rate to air can be compared. The hot dog is shown next to a common polartec fleece glove. Two hot dogs were compared- one bare and the other wrapped in a single layer of saran. The saran prevents evaporation, just like the disposable glove. The hot dog was inserted in the thumb pocket, and placed in a -8F freezer that does not include a built-in fan, so the air is stagnant.
As you can see, even though both hot dogs are sealed inside the glove, the fleece is moisture and air permeable. The bare hot dog evaporates water which then passes through the fleece and cools faster than the saran-sealed hot dog. So wearing a nitrile inner glove will keep you warmer. We also mounted a small fan above the gloves that produced a 5 m.p.h. wind flow. Wind blows away the stagnant bubble of insulating air surrounding the glove. Even if both hot dogs were sealed in saran, wind cools your fingers faster. Which is why you really should wear an inner, mositure impermeable glove inside a mitten on cold windy days. 4This curves hides an interesting piece of physics. The towel freezes in the first couple of minutes, so why would the fan continue to evaporatively cool, if the water in the towel is solid ice? Shouldn't the "fan" curves quickly approach the stagnant air curves, once all the water is frozen? It turns out even solid ice molecules can thermally evaporate, forming a cold "fog" over the frozen towel which will be blown away by the fan. Ice vapor pressure (a measure of the number of molecules that thermodynamically gained enough energy to evaporate), drops in half from 50F to freezing, and another factor of two from freezing to 20F. So plenty of water vapor molecules linger near the surface to create a strong wind chill effect- even below freezing. You may have noticed this phenomena in winter- snow hidden in the shade disappears on days which never break 20F. Or food desiccates in the freezer after a few months. Called "sublimation", its a vital link in the understanding our climate. 5 The reverse is also true. For example, if you forgot to defrost your turkey in the fridge three or four days before Thanksgiving, try placing it (still wrapped in the watertight plastic bag from the store), in a bucket of ice water. Yes, ice water. Because of improved thermal contact, ice water defrosts faster that air in a fridge- perhaps taking one day instead of three. You can even run the bird under hot water for a few minutes, remove the plastic bag, and dunk the frozen bird directly in the brine. To avoid bacterial growth, just make sure the water always has ice floating around the bird. 6You might wonder why the cooling curves in air are not simple smooth exponentials. There are a couple of factors at work. First, the freezer itself has a small circulation fan, and when it turns on (sometimes every ten minutes, sometime every half hour), the increased air flow slightly changes the cooling rates. Second, while the thermocouple is located in the center of each bottle, water stratifies in density by temperature, water freezes from the bottle wall inward driving air bubbles to the central core, and there is a bit of convection along with temperature inversions. So the thermocouple measurement varies in time as these temperature gradients sweep by. |
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |

 It's for exactly this reason that commercial "blast freezers" are so effective- a large fan forces cold air across the food like an arctic jet stream. Food cools in minutes rather than hours.
It's for exactly this reason that commercial "blast freezers" are so effective- a large fan forces cold air across the food like an arctic jet stream. Food cools in minutes rather than hours.