| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| spray and pray |
|
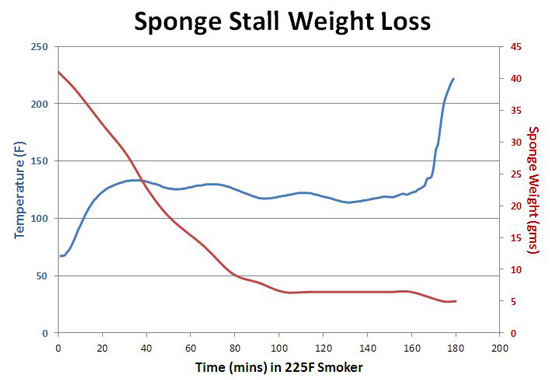
Heat tends to desiccate meat, so it's tempting to add moisture during cooking, hoping the added liquid will somehow be absorbed, resulting in a more succulent meal. One traditional approach is basting. Another is creating a Swedish sauna by placing a water tray beneath the food. Or directly, by simply spraying the meat with water or a marinade, and praying for the gods of juiciness to suck up this sweet offering. On the one hand, spritzing a fraction of an ounce of liquid into an oven or onto the meat is more or less like spitting into a hurricane. It can hardly be expected make a dent in the humidity. And, a water spray is more likely to drip off the shiny, oily, crispy bark than be absorbed. On the other hand, small amounts of moisture evaporating from WITHIN the meat, while baking at low temperatures, can be a remarkably effective coolant. As a baseline, we'll test two elementary surrogates, avoiding the complexities of real meat proteins and chemistry. The first is nothing more than a square cellulose sponge- about 1.75" on a side weighing 7 gms dry. I moistened the sponge to reach 40 gms (placed in a bowl with water, and squeezed out), then cooked in an electric smoker at 225F. Using the rather primitive but accurate apparatus shown above1, I could simultaneously measure its weight and its temperature. The results are graphed below:
You'll note the wrinkles in the curves- this is simply a reflection of the electric smoker's thermostat cycling on and off during cooking. More importantly, note how the sponge's temperature (blue curve) stalls around 135F. This directionless plateau will annoy anyone but the most relaxed backyard barbecuer, for whom dinner is always just one more beer away. As we've explained in other articles, the stall is a result of evaporative cooling. However, the amazing part of this graph is the red curve which measures the sponge's weight. With only a few grams of water remaining out of the original thirty three (e.g. see 100 mins.), it managed to supply enough cooling power to plateau for almost half the stall period! Until the last drop of water was gone. Clearly, the latent heat of evaporation is enormous. I stumbled upon another illustration of the power of evaporation while assembling a faux meat tenderloin. The purpose of this fake meat slice was to establish a baseline, demonstrating how quickly an organic mass, the same size and shape as a piece of meat, would heat up if it lacked juices and could not cool during baking. The faux tenderloin is a cup of canola oil. However, unlike a solid piece of meat, if you heat a liquid, convection currents would rapidly transfer energy more quickly than diffusing through fibrous muscle. So, I filled the oil with dry sand from a bag in my shop. The sand, like muscle, blocks thermal currents. Surprisingly, that sample (red curve) did not simply heat up like a hunk of steel. Instead, it almost stalled, only gradually approaching the oven temperature of 225F. I then constructed a second faux tenderloin, this time after drying the sand for hours in a 500 F oven before adding to the oil. The result is shown by the green curve, which behaves like a solid mass. Apparently, the little moisture hygroscopically adsorbed on the sand was enough to slightly evaporatively cool through the oil, delaying cooking2. Now let's graduate to meat- real pork tenderloins about 3" in diameter and 2 lbs in weight. Tenderloins, despite their name, are less juicy and less tender than many other cuts, such as pork shoulder. So, they rarely ooze enough juices to stall completely, unless the oven is very humid. But they are very uniform. In this experiment, I placed a tenderloin in a kitchen oven and recorded its internal temperature over a few hours of cooking, without peeking. I then removed this "control" sample, and inserted a second tenderloin of the same size and shape. Only this time I sprayed the meat every half hour, being careful to just briefly open the oven door so the oven temperature recovered quickly. The spray was a mixture of five parts apple juice to one part apple cider vinegar. And here are the results at three different temperatures: At 325F and 250F, spraying had no effect on the cooking time (the 325F sprayed results are hidden under the black plain curve). And no real effect below 140F internal temperature- the meat has plenty of its own juices supplying moisture- for a while. Not surprisingly, the final weight losses were very similar. But, at 225F, spraying does slightly cool the meat, extending cooking time by around an hour out of ten. The reason is simple- at high temperatures, water evaporates quickly and doesn't coat the meat long enough to evaporatively cool for the entire half hour. At lower temperatures, the evaporation rate and stall temperatures are reduced and more closely matched3. This extra humidity and surface cooling enables the meat's juices to linger just a bit longer inside. But again, not a huge difference. This trend is amplified by cooking slightly below the boiling point. As you can see, spraying has a dramatic effect at lower temperatures (the tiny wiggles mirror the oven's thermostatic cycling or the slight cooling immediately after spraying). But few people have the patience or equipment to barbecue this low: These experiments were all carried out in a kitchen oven, where there is minimal air flow (around 5 air exchanges per hour). However, as any athlete knows, evaporative cooling is more effective in a breeze. So I also evaluated spraying in a pellet smoker, where air is exchanged more than 100 times an hour. In this case, both the sprayed and plain tenderloins were tested simultaneously, so any smoker temperature reductions while "peeking" would be in common. But it had no effect at all. Although high air flow rates rapidly cool, they also dry out the sprayed surface layer just as quickly. I could detect a larger effect by spraying the ribs concave side up, forming a "bowl" to catch the sprayed liquid. Or, if the surface was coated with a slightly sticky barbecue glaze, which held on to moisture. Still, as you can see from this experiment on small cuts of brisket at 260F, there is little difference in cooking time between glazed, sprayed or simply rubbed surfaces. Weight loss and tenderness were also similar, with a slight edge with glazing. In a similar experiment on chicken thighs, the rubbed or foiled chicken lost 33% of its initial weight, while the sprayed lost 29% and the glazed only 22%. Of course, foil wrapping traps moisture and leads to fork tender meat, but sucks out flavor, so its a pyrrhic victory over dryness.
So does spraying make a difference during cooking?
In the end, spraying is probably a harmless technique in the right hands, and more likely a flavor disabler in the wrong.
|
|
--------------------------------------------------------------------------------------------------------
1 To measure such small weight changes, I suspended the sponge from a thread hanging through the vent hole of the electric smoker. The string was attached to a cantilever with one end resting on an accurate digital scale, and the other on a fixed block. The thermocouple wire was inserted into the middle of the sponge, and carefully wound to adjust any tension on the sponge to zero. Finally, the scale was calibrated to adjust for the mechanical disadvantage of a second class lever. I could accurately detect a half gram shift. 2 You might have noticed the cellulose sponge stalled at 135F, while the humid sand faux tenderloin and real meats curves didn't even bend over until 165F. The reason is surface area. The sponge, like a car radiator, has an enormous capacity for cooling due to its complex and extensive surface texture. So a little moisture goes a long way. 3Personally, I cook tenderloin to an internal temp of 135F to140F, so spraying has little effect at any temperature. |
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |