| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| to the bone | ||||||||||||||||||||||||||||||
|
April 2013 Life would be so much easier if just a few simple rules applied to every situation. And with cooking, sometimes they do. You should always salt meat an hour or two before cooking- the salt helps retain moisture despite the intense heat of grilling. When baking, you should always portion ingredients by weight and not by volume- measuring cups are an abomination of approximation from a digital-scale-free past1. And don't eat anything bigger than your head. But when and where should you measure temperature during cooking? Common sense advice seems as scattered as it is adamant:
Unfortunately, no two cuts of meat, or two species, or two ovens are ever the same. While the laws of physics are constant, how they translate into cooking advice may seem arbitrary and capricious at first. But as we will discover, a common, though invisible, thread unites them all. Even in the face of a clever and adaptable natural world.
Thermal conductivity is a measure of how fast heat passes through a substance. Wood impedes heat flow, copper enables it. Wood has a low thermal conductivity, copper a high one. Which is why a frying pan's handle is carved from wood, while the grilling surface is formed out of copper. Fat, inch for inch, is two to four times faster at conducting heat than fur or pelt, because these soft coats entrain air2. Air is a terrible thermal conductor, and nearly stops heat flow in its tracks (for the same reason, air is the insulator in fiberglass wall batting and sleeping bags). Animals would be much warmer growing a thicker pelt, than a fatter belly. Primarily, fat is deposited as a food larder to tide the animal over during the winter. And they need lots of food to survive, so the layer of fat is thick, and at the beginning of the winter, often matches fur in its absolute insulation value. If a walrus waddled out of a freezing cold ocean onto a hot sunny pebble beach, it would quickly overheat. Too well insulated. But nature has evolved a clever trick-- its fat is infiltrated with a dense network of capillaries, transforming its skin into a blubbery radiator. On a hot day the walrus shunts blood to the surface, cooling off, while in cold water, the capillaries shut down, allowing fat to behave more like a winter parka. In a live animal, fat is part of an active thermoregulation system. But a brisket from a dead cow couldn't shunt heat away from the grill unless it could also stand up, moo and amble away. Instead, a dead fat layer is measured to have twice the insulating value of meat, at best. So it will deter heat, slightly, but perhaps not as dramatically as people believe. And it doesn't stop there. As the meat cooks, up to 10% air replaces oozing juices. Meat's thermal conductivity is dominated by its water content, and as it dries out, lowers its thermal conductivity. Sometimes impressively:
So fat is a modest insulator compared to raw meat. But, after six hours smoking a brisket in the dry air of a smoker, the meat and the fat cap quickly approach each other in thermal conductivity. Making a simple rule of thumb a matter of circumstance, not convenience.
Once again, nature is too clever and too diverse to summarize with a simple yes or no reply. Roughly speaking, bone consists of an outer hard dense layer (compact or coritical), surrounding an inner foam-like layer (porous or cancellous). The porous layer is often infiltrated with marrow which functions as a food storage and/or blood producing organ. In a cow, the leg bones are thick with strong, compact bone. In a bird, lightweight porous bones are the norm to reduce weight for flight. And these bone types can vary wildly in thermal conductivity. For example, compact bone conducts heat about one third more readily than porous bone. Except when it doesn't. Why "except"? Well, in a hunk of meat fresh from the butcher, the bone is generally sliced clean through on a band saw. The marrow may or may not slip out, or melt out during cooking. And, if the cut meat is shipped to your butcher in a cryovac plastic bag, water and meat juices will infuse the porous bone, dramatically raising its thermal conductivity. So a piece of bone might conduct heat faster than meat, or slower. Depending. Now let's apply this perspective when cooking three different kinds of meat- a steak, a bone-in pork shoulder, and a whole roast chicken. How quickly the meat heats up, and which part cooks fastest, depends partially on the thermal conductivity. If the conductivity is high, then heat will rapidly diffuse in from the surface to the interior. But not uniformly- the heat would detour around a low thermal conductivity region.
As an analogy, consider this cascading fountain. The wider the spout, the faster water flows out from each bowl. This is like thermal conductivity. But, the deeper each bowl, the longer it takes for each to fill and spill over to the next level. This is the heat capacity. The equations of heat flow combine both properties into a single metric called the "thermal diffusivity", which is equal to the the thermal conductivity divided by the volume specific heat capacity. The higher the thermal diffusivity, the faster things heat up. Because thermal diffusivity is a ratio, a highly conductive material with a larger heat capacity, might heat similarly to a material with low conductivity but low heat capacity. After combing through over 50 scientific papers, I selected the most representative values for these three properties (bone 50:50 is half porous, half compact filled with marrow). These are averages, and there is a remarkable +25% variation depending on species, the specific muscle, pH, age at butchering, storage conditions, type of feed, and so on.
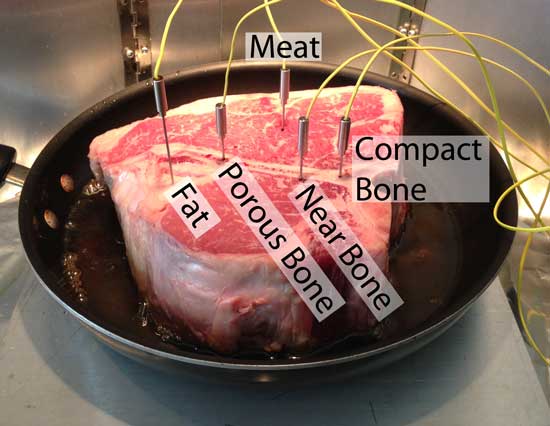
The first thing to notice is how little the average diffusivity varies between fat, meat and bone. Factoring in the broad range of properties characterizing food in the real world, bone should not be type-cast as an insulator. Nor as a heat conductor. In fact, the shape of the bone and it's placement in the meat, are often more important. Let's begin with a steak- a 3" thick porter house, bone-in. I drilled 1/16" holes in the porous and compact bone, inserted thermocouples at various locations, and seared from one side on a 300F fry pan:
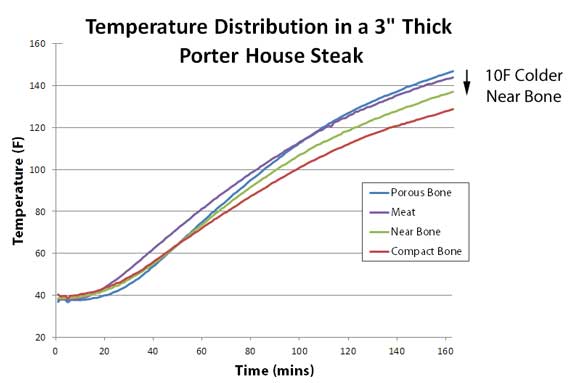
The pan was placed on a 1" thick aluminum heat spreader over a gas burner, and there was 1/8" of oil in the pan to provide uniform thermal contact between the meat and the pan. Temperatures were uniform to +5F. The thermocouples were inserted to identical depths, around 1.75" above the pan's surface3. And here are the results. Note meat adjacent to the bone (red curve) ran around 10F colder than in the center of the steak (purple curve). So in this case, avoiding the bone when measuring the steak's temperature is good advice. It's the difference between rare and medium rare. Interestingly, the porous bone (blue curve) cooked faster than the compact bone:
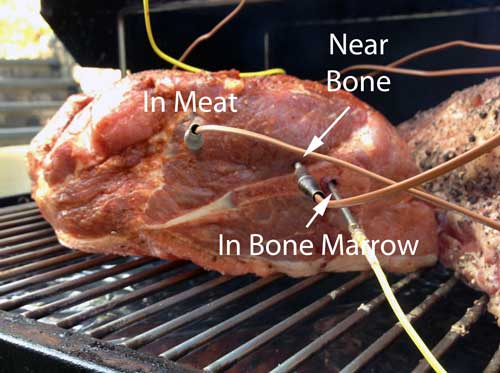
And the fat? It turned out to cook at almost the same rate as the meat itself- the two curves practically laid on top of each other. So the bone makes a small but important difference, at least locally. Yet this small effect is often overwhelmed by other factors. Note I cooked the meat in a thin pool of oil, so no matter how the meat's surface curled, it remained in solid thermal contact with the heat. But I also ran the experiment without oil. In this case, the meat shrank back after a while, exposing the T-bone, cantilevering the steak over the pan on a bone tripod. Since the bone was now in optimal thermal contact with the griddle , the bone ended up around 15F hotter than the meat! Yet not all bones are created equal. Many pitmasters cook their pork shoulders "bone in". Some hope to add flavor (a very minor effect it turns out4), while others expect the bone to speed up the cook. So what is the reality? I instrumented an 8 lb pork shoulder with three thermocouples. Each 2.25" deep, and around the same distance from the from the surface. One was impaled in the meat, one adjacent to the bone, and one in the bone marrow itself:
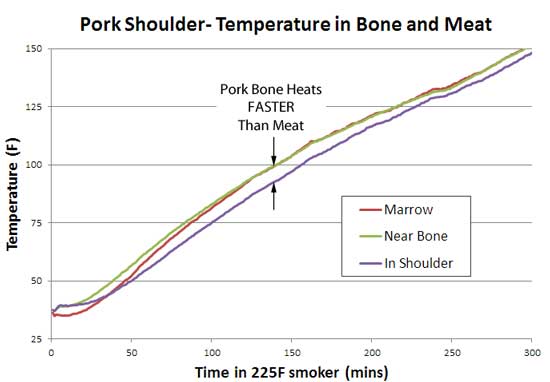
It turns out pork bone conducts heat faster than the meat. Not by a lot- the temperature difference between the bone and the meat was about 7F at its greatest, and narrowed to zero at the stall (around 158F), and later in the Texas crutch. From analyzing the initial heating rate -- where the meat near the bone cooked fastest-- it is clear the bone has the largest thermal diffusivity, carrying along the encased marrow and nearby pork:
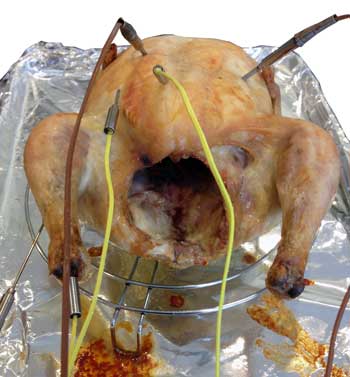
To my mind, this is a tie, especially over a half-day cook. Finally, a whole roast chicken. I drilled a hole in the "ankle" of the chicken leg and inserted a thermocouple up to the middle of the bone. Other thermocouples were located throughout the bird. As you can see, the cavity is empty and open to oven air.
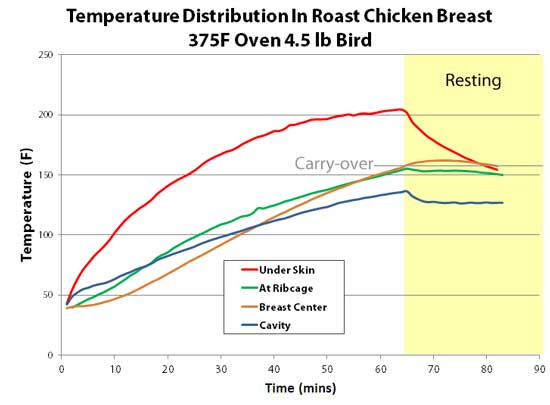
The chicken was roasted in a 375F oven on a wire rack until the center of the breast reached 150-155F. The bird was then removed from the oven and allowed to rest for 20 minutes. Pretty much my normal cook for a tender and juicy roast chicken. The chicken breast plot tells an interesting story. Let's start with the red curve, recorded by a thermocouple just 2 mm below the skin. Note it heated up the fastest- not surprisingly, as it was closest to the hot air. In under an hour the top few mm's reached 212F- dramatically overcooking the white meat. But it never approached 375F- evaporative cooling stalled the moist breast's temperature at the boiling point . Then, during resting the same skin cooled off fastest, again not too surprisingly, as the skin was closest to the cool kitchen air.
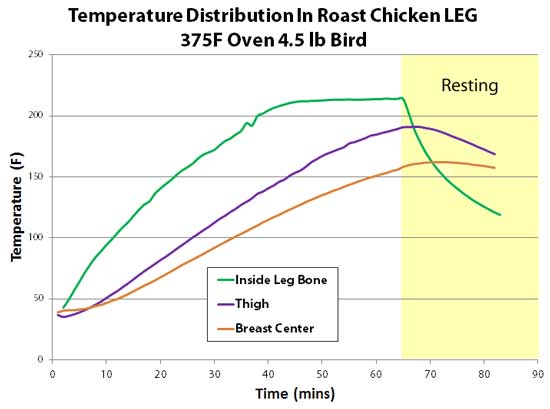
Now let's examine the blue curve, measured at the dead-center of the chicken's cavity. This area rose the slowest, only reaching 135F in an hour. True, one end of the cavity is open to the oven, but the other end is nearly sealed by a tiny 1" diameter neck opening. As the cavity is horizontal (unlike a vertically roasted bird), convection is suppressed, stranding a cold moist bubble of air within the interior. So the coldest part of the bird is the hollow interior. Consequently, the rib cage thermocouple (green curve) shadowed the cavity temperature- warmed just a few degrees higher by heat trickling in through the breast meat. Then there is the breast meat itself (brown curve). The thermocouple was inserted to the center of the thickest part of a very ample chicken breast, which is closer to the neck region than the middle rib. Ah, the miracles of selective breeding. So, the white meat was thinner above the rib cage's thermocouple. And they ended up reaching 150F at the exact same time. So there is no issue measuring temperature near a rib bone vs the breast center5, at least cooked in this specific manner. And finally there is the leg. I drilled a small hole through the ankle and threaded a thermocouple into the middle of the leg bone. A second thermocouple was placed in the center of the thickest part of the thigh (in this bird, located on the bottom rear of the bird). Because I elevated the bird above the baking tray (without the cooling effect of a bed of roasting veggies), and the tray was seated in the bottom third of the oven near the heater, when the breast hit 150F, the thigh reached 180F. In other words, both cooked to perfection. The leg temperature (green curve) reached 212F in only 45 minutes- even faster than 2 mm under the breast's skin! Chicken bones are relatively porous and filled with marrow (which is basically fat), and are slightly poorer conductors of heat than chicken meat. But, the dangling leg bone is heated from all four sides, and conducts energy down its length. So geometry trumps diffusion, and the bone wins the race6. As the bird rests, the same geometry turns the leg into a radiator, and the bone cools rapidly.
Thus a bone can impede or accelerate cooking. Fat may or may not be an effective insulator. Depending on, well, everything discussed above. Meat's thermal properties are not very uniform or even predictable, so the trick in cooking is to outsmart the laws of thermal diffusion, by:
|
||||||||||||||||||||||||||||||
|
-------------------------------------------------------------------------------------------------------- 1 In fact, sometimes you should weigh DURING cooking, it's often the best predictor of when baked goods have achieved just the right blend of crustiness and moistness. 2 Note the fat thermocouple was longer than the other four, so I could probe at different depths post-cooking. But it was inserted at the same depth as the others during measurement. 2 Deep-diving mammals have little or no fur, because insulating air would be squeezed out by the high water pressures. Which again illustrates why a blanket categorization of fat vs. fur is unwarranted. 4 In a stew, bone marrow definitely adds flavor and a silky texture to the gravy. But in a short cook, like a grilled steak where the unctuous marrow is blocked by 1/4" of solid bone, taste and dye tests prove there is almost no flavor advantage. 5 Among all four curves, only the breast meat continued to rise in temperature during resting (e.g. "carryover"). The breast is thick enough to retain heat, and during resting, some of the 212F surface heat continues to migrate towards the center, raising the breast by around 5F. 6 Unlike steak, the color of chicken meat near the bone may stubbornly remain pink, even when fully cooked. Chickens bones are thin, so the pink marrow is visible. And dark meat contains large amounts of pink myoglobin. One reason people incorrectly assume the temperature is colder at the rib cage or near the thigh bone. See this article at AmazingRibs.com for more details. |
||||||||||||||||||||||||||||||
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |
 Let's start with fat. We all vaguely recall watching a PBS nature show documenting the animal kingdom's cruel battle for survival through a long and cold winter. Species such as the walrus or bison build up thick layers of blubber or fat, which "insulates them" from the cold. Except, it turns out, fat isn't a great insulator at all, and if it were, the animals would surely die.
Let's start with fat. We all vaguely recall watching a PBS nature show documenting the animal kingdom's cruel battle for survival through a long and cold winter. Species such as the walrus or bison build up thick layers of blubber or fat, which "insulates them" from the cold. Except, it turns out, fat isn't a great insulator at all, and if it were, the animals would surely die.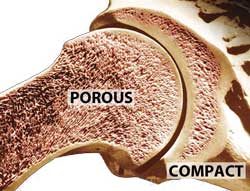 If not fat, what about bones? Do they conduct heat efficiently, or act as an insulating barrier?
If not fat, what about bones? Do they conduct heat efficiently, or act as an insulating barrier? More critically, heat is not merely passing through, but warming the meat as it progresses. For a given unit of heat (e.g. a Joule or a Calorie absorbed from hot oven air) some materials rise in temperature rapidly, while others take their time. This is called heat capacity, and the higher the heat capacity, the longer it takes for the temperature to rise.
More critically, heat is not merely passing through, but warming the meat as it progresses. For a given unit of heat (e.g. a Joule or a Calorie absorbed from hot oven air) some materials rise in temperature rapidly, while others take their time. This is called heat capacity, and the higher the heat capacity, the longer it takes for the temperature to rise.