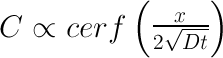| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| follow the salt |
|
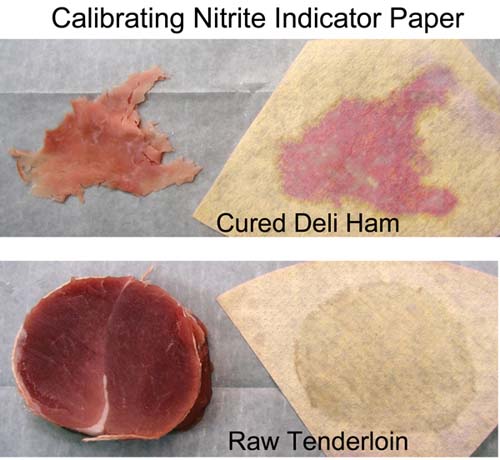
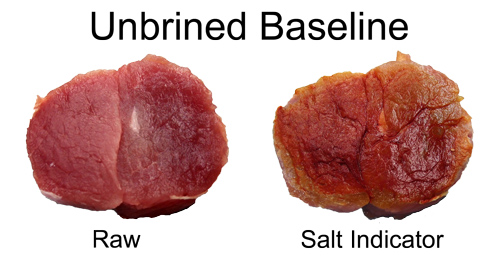
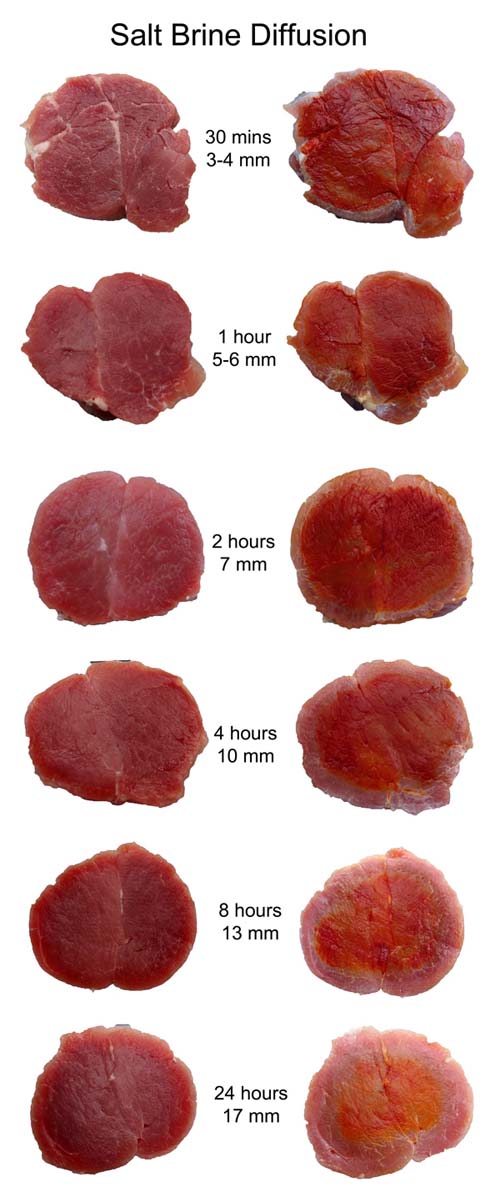
November 2011 Salt is a traditional preservative and flavoring agent, although today, with advances in food transport and packing, its preservative powers are increasingly a thing of the past. Indeed, health concerns have stimulated a decade's-long effort to reduce the concentration of all salts in meat production. Still, recipes often suggest brining or rubbing meats with salt to tenderize and flavorize (but not, as it turns out, to moisturize), and in the case of curing salts like sodium nitrates and nitrites, to create a pink color as well as preserve. But how can we get the salt into the bulk of the meat? In ground products, like sausages, the salts are mixed into the meat before stuffing into casings. No mystery here. But in solid cuts of meat, like bacon or ham or a brined chicken breast, the salts are rubbed or brined on the exterior yet somehow make their way into the center. How does this magic occur, and how long does it take? How long should you brine for? In turns out when salts are exposed to water, they dissolve into charge atoms (called ions). These atoms are very tiny and mobile and can easily diffuse from the surface past the meat molecules, given enough time. Heating the meat adds energy to all the atoms (including the ions), as well as dissolving some of the cell membranes and fat deposits, clearing out more space for the ion's travels. So diffusion rates generally increase at higher temperatures. The ions have no particular "sense of direction", and are merely spreading out in all directions because they are constantly being jostled by their environment. This so-called "random walk" will eventually distribute the ions uniformly through out the meat. To find out how long salt diffusion takes in a realistic cooking situation, let's experiment with curing salts on pork. I rubbed a mixture of sugar and sodium nitrite on the surface of a 2 lb pork tenderloin cylinder, at about 150 mg of NaNO2 per pound. This is about 2x the recommended levels, but wanted to be sure there was enough salt to be detected. After rubbing, the meat was wrapped in saran and refrigerated for an hour. The surface was then washed off, dried and the tenderloin was then cooked in a 230F oven. As the temperature rose, I periodically cut off 2" slabs of meat from one end to measure the salts progress towards the center. Unfortunately, while you can distinguish subtle changes in the meat's transparency near the surface after rubbing or brining, and easily discern the margins of a pink cured ring, you can't be sure these features reflect the underlying salt distribution. For example, after a few hours submerged in plain water, a 2 mm thick, gray mushy surface layer develops, (even without the influence of added salt). Fortunately, chemical indicators which change color in the presence of nitrate and nitrites are available. These indicator chemicals are easily obtained at any pet store, since its important to measure nitrate levels (from fish pee) in an aquarium. For more on how I modified these aquarium kits for use in the kitchen, see below2. For now, note we fabricated sheets of color indicating paper, which we pressed on the meat and then peeled off. The filter paper absorbed meat juices containing salts, and turned pink in their presence. To roughly calibrate this method, I pressed a shred of deli ham on the paper. Note the pink pattern matching the shape of the ham. I also pressed a 2 1/2" diameter slab of raw, uncured pork on the paper. Other than a clear shadow from the juices, there is no pink signal. So far, so good:
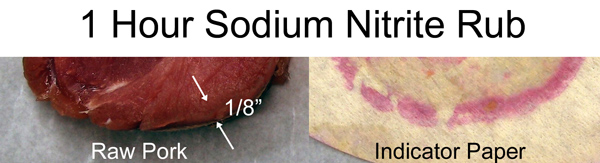
Before cooking, I cut off a slab of the brined pork. Typically, there is a slightly more translucent layer a few mm's thick near the surface after brining. The indicator paper confirms this layer contains nitrates. Basically, after an hour in a cold fridge, the salt diffused in only a tiny distance (see these similar results on pork revealed with dye).
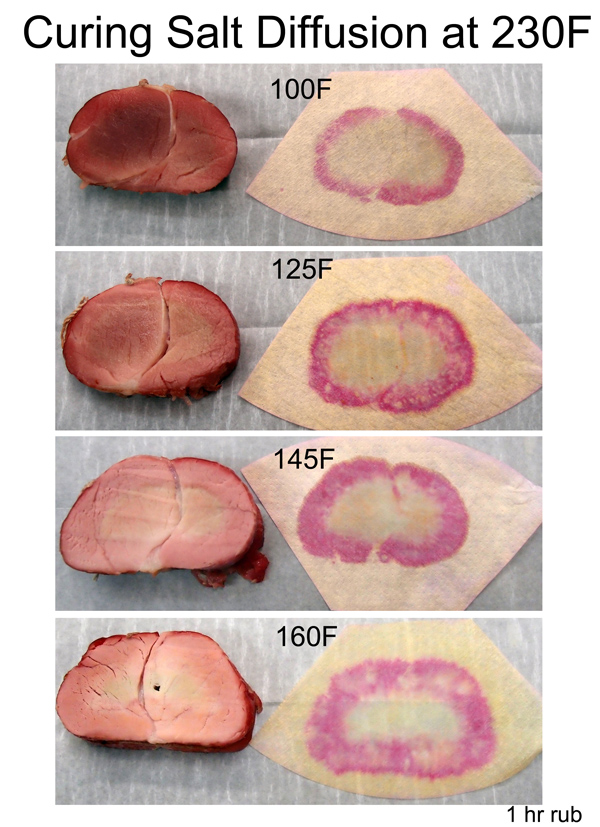
The meat was then baked in an oven, and monitored periodically over the next three hours. At these higher temperatures, diffusion rates increased and the salt diffusion front steadily marched away from the surface:
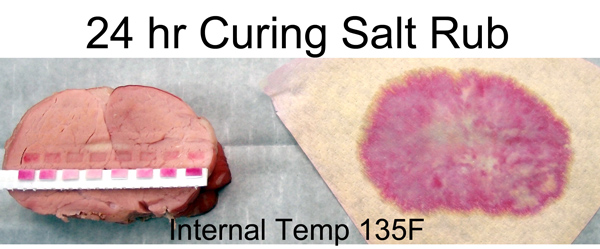
Even at 100F you can just make out the faint beginnings of a cured pork ring. Note how the ring's growth is relatively slow, despite the additional time and higher temperature. Diffusion is an exponential process2, with the most dramatic movement early on. This is why a 30 minute brine is nearly as effective as a two hour soak3. Alternatively, you can dry rub brine for an entire day. I repeated the experiment as above- here is a cross-section at the 135F point. Note the pink ring almost penetrated all the way to the center. Even though the majority of ions are still working their way forward, the exponential tail of a diffusion process suggests a small fraction of the ions have travelled a long distance in a short time. Enough to produce a pink ham color. But, as the indicator papers demonstrate, the actual concentration ring is under an inch thick at this point.
From these experiments we have discovered how salt moves through the meat, and expanded on the general brining rules offered by most cookbooks:
|
|
--------------------------------------------------------------------------------------------------------
1There are numerous over-the-counter color indicator kits available to measure nitrate/nitrite levels (due to oxidation, both chemical forms are present, even if cured in pure nitrite). Presumably, they use some form of the Griess reaction. I've modified two of these kits. First, I cut the little indicator pads off strips made by Waterworks Industrial Test Systems (left photo) and reassembled onto a longer stick (see 24 hr image above). This pad array produces a crude 1D image, but has the compensating virtue of accuracy because the pads are still intact.
The second method was used throughout this article. Not as precise, but excellent for 2D visualization of nitrates above a threshold level. This kit (made by API) normally indicates a color change in a water-filled vial. Instead, we re-purposed the same chemicals to activate the filter paper, following this procedure:
I also tried a third method requiring no special equipment (useful only after the meat reaches 145F). Simply cut out little meat plugs and taste- your tongue can easily detect salt, even at these low levels. All three methods are in agreement... 2 In principle, an accurate model of ionic diffusion would apply physical laws in a cylindrical geometry (the tenderloin roll), compensate for the temperature dependence of the diffusion constant "D" as the meat cooks, adjust for the flow of juices and denaturing of proteins, and account for cell wall transport and minor osmotic pressure rupturing. Too complex. Fortunately, concentration gradients are low, the nitrite levels are diluted after the first few minutes (under 100 ppm), and the meat is large compared to the size of animal cells. Averaging should apply, and simple diffusion, often known as "Fick's Law", can be our qualitative guide. So, consider a standard one dimensional diffusion model in the case of a fixed supply of surface ions. "C" is the concentration of salt ions in time and space. Initially (at time t=0), all the nitrite atoms are found on the surface ("x" is the distance from the surface). The solution is a simple Gaussian:
I've plotted the salt concentration for different time periods using this equation. All the units are relative- we are just looking for its qualitative behavior. Note at a mere t=0.05, the surface salt concentration has rushed into the meat creating a thin but discernible layer (the RED curve). This initial behavior mimics the few mm layer observed after immersion in brine. Then, at MUCH longer times t=0.5, etc, that layer begins to spread out and become more diffuse. The indicator filter paper loses sensitivity below some concentration, so can produce the impression of a sharp cut-off at some distance out from the surface. In fact, the salt distribution has a long tail that quickly drives a small fraction of the salt ions all the way through the meat. But most of the ions lag far behind. I believe osmosis is a less critical path for ion movement than pure diffusion within the meat juices. Meat contains a network of veins, capillaries and intramuscular juices enclosing a significant fraction of its total "free" water. A superhighway for ion diffusion. Strictly speaking, osmosis is the movement of a solvent across an ion-selective membrane where there is more solute on the other side to dilute. Osmosis is normally analyzed in the chemical language of solvents (water), solutes (salt ions), partial pressures and the like (I've always thought the similar nomenclature to be confusing to my students, and wish it wasn't standard in the literature). In reality, both diffusion and osmosis can and do occur together. One can think of this osmotic pressure barrier as a reduction in the effective diffusion constant, and by averaging over many interconnected cells, still conclude a Gaussian process is an accurate model. 3 We also measured table salt diffusion DURING wet brining, again modifying a standard water quality testing kit. In this case, the salt brine was a 6% solution by weight, and the foot long pork tenderloin was brined in the refrigerator over a 24 hours period. Disk shaped samples were periodically sliced from the pork and tested for salt using a Taylor Sodium Chloride Drop Test Kit K-1766, by converting silver chromate (orange) to a silver chloride (white) following method show in this video: MAKE SURE TO WEAR GLOVES, GOGGLES and DISPOSE OF USED CHEMICALS PROPERLY!
At these high levels of reagents, the test color will be clear in the presence of high levels of salt, brown-red without salt, and light yellow-brown for low salt concentrations. As a baseline calibration, note how the unbrined pork is colored dark brown to red over most of the surface, reflecting the low salt level in raw pork.
And here is the result over the a period of a day (left image is the brined meat slice, right image the same slice treated with the chlorine imaging solution):
In addition to the obvious movement of the salt diffusion front over time, the color of the indicator becomes lighter as the brine progresses, indicating a transition from an absence of salt in the center, to a low concentration- just as expected from diffusion (although in the case of diffusion from a brine rather than a rub, the supply of salt ions at the surface is unlimited, so the curve is actually a complementary error function rather than a Gaussian). I then plotted the position of the actual salt "front" over time, and compared it to a constant penetration rate of 4 mm/hr (the actual speed during the first hour). Clearly, as with any diffusion process, the rate of penetration slows over time. Which is why an hour's brine is nearly as effective as a half day's brine. And why doubling the brining time doesn't double the depth of penetration.
The inward diffusion also explains why a salted steak dries out after first becoming glossy. The usual explanation - a two step process where the salt "draws" water out of the meat, forms a slick brine, and the brine is then re-adsorbed by the meat, is misleading. Salt is hygroscopic, which is a fancy way to say it adsorbs moisture from the environment. This is why salt in salt-shakers often bonds into a concrete-like "cake". On a humid winter day, you may have noticed rock crystal salt, scattered on a sidewalk, apparently "attracting" a ring of water out of thin air. This is a misnomer- the salt does not emit some kind of force field that reaches out and calls water home, like some ionic Pied Piper. Actually, ever-present water vapor molecules in the air and concrete randomly encounter the salt, then stick to it rather than bounce off. Why does it stick? Well, water (a "V" shaped molecule), has its negatively charged oxygen and positively charged hydrogen atoms slightly displaced from each other on the tips of the "V". In most compounds, the atoms are symmetric and there is no net charge. But in the case of water, this "polar" asymmetry creates a local electric field around the size of the water molecule itself. When an air vapor molecule stumbles in very, very, very close to the NaCl crystal (like a golf ball on the rim of the cup), it drops in. The salt feels the attraction of this weak electric field, grabs the water, and then breaks the salt apart into a positively charged sodium ion and a negatively charged chloride ion. Water molecules are captured on the surface of the crystal (or even by a concentrated brine), and eventually, accumulate into a pool of glossy liquid. The polar nature of water is why it's practically a universal solvent. This hygroscopic effect is easily observed. Here I suspended a 0.15" salt crystal very slightly above a cucumber slab. Water vapor constantly evaporates and is readsorbed by the moist cuke, creating a humid boundary layer just above the surface and within reach of the salt crystal. The salt acts like fly paper, catching any insect that lands on its surface. After an hour, the salt facets are slick and moist, forming a briny droplet on the bottom face. The cuke's surface immediately below the droplet actually shrinks away into a bowl-shaped depression as the the cuke desiccates. Finally, the brine drips down, touching the cuke's surface to form an hour-glass meniscus.
Or watch this movie of the droplet forming- a small depression is visible below the drop after it falls:
So what happens when you shake salt on meat, as seen in the montage to the left (courtesy of AmazingRibs.com)? Well, a tiny quantity of juices loosely bound in the meat, as well as humid air near the surface, are hygroscopically absorbed by the salt. A thin slurry results. This layer continues to build until all the salt is dissolved. If there was a pre-exisiting layer of water on the surface, the salt would dissolve faster, but a layer of water is not required. Simultaneously, salt is diffusing into the meat, and would do so even without the surface brine. No osmotic pressure or an "attraction" of water from the meat- the same phenomena occurs on a concrete sidewalk. Eventually, the salt diffuses fully into the meat, and some of the moisture follows along, trapped by the water's polar charge. Most of it simply evaporates. It's not a two-step process, though appears to be so due to the slow rate of dissolving salt crystals. Simply diffusion and hygroscopic surfaces working in parallel. You should always salt your meat about 1/2 hour before use, so the salt has left the surface and started working its way inside. Otherwise, it doesn't have time to help retain moisture. About 1/4 to 1/2 tsp per pound is best- especially on burgers, where too much salt will gel the meat proteins and make for a dense patty.
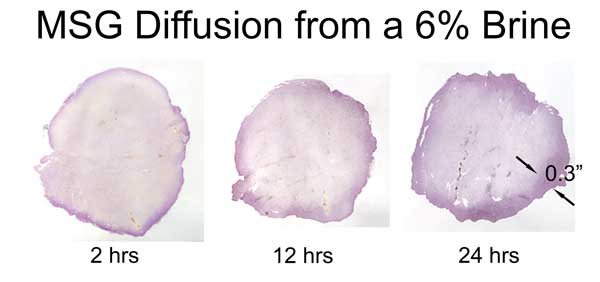
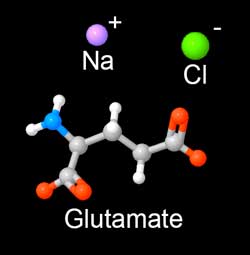
The "glutamate" molecule is an amino acid which naturally occurs in the body and is found in many foods. It aids metabolism and helps eliminate the waste products of digestion. So any assay must be able to distinguish MSG absorbed in a brine, from naturally occurring amino acid levels. We discovered that ninhydrin dye is an effective assay. Ninhydrin turns purple (Ruhemann's purple) upon exposure to most amino acids. In addition to its use as a laboratory assay, ninhydrin plays a staring role on CSI detecting fingerprints, by reacting with amino acids from skin oil left at the crime scene. It will also turn your hands bright purple, if you don't wear gloves...... In this experiment, we brined pork tenderloin in 6% MSG (by wt). At 2, 12 and 24 hours slices of meat were removed and tested with ninhydrin. The protocol is relatively simple:
As these images reveal, MSG diffuses about a third as fast as table salt. Also, the diffusion front is ragged and not uniform. Unlike salt ions, the glutamate molecule is impeded by thin layers of fat or silver skin. So, if you add MSG to ribs and brine overnight, the entire rack is fully saturated. But not so in a big brisket. Sprinkled on a steak just before grilling, MSG adds flavor only to the surface- but each bite distributes the MSG throughout your mouth- fooling the brain into thinking the entire steak is a prime cut. BTW, the initial worries over MSG causing headaches and "chinese restaurant syndrome" have been studied and thoroughly disproven. What about sugar? Many people add sugars to brines, for flavor and color. The sugar molecule is larger than a salt ion, and using similar techniques, we've demonstrated that diffusion levels are 5-10x lower than with salt. Which will help brown skin or bark, but does not add much flavor beyond the first few mms.
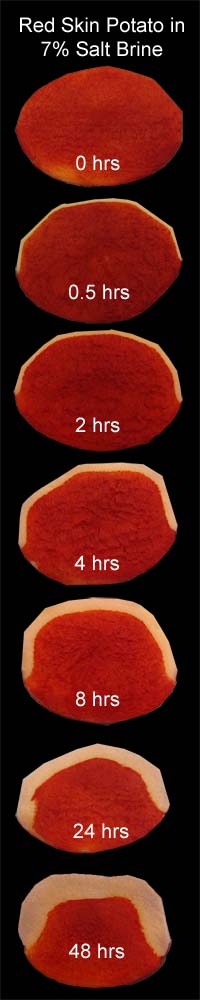
A similar diffusion phenomena occurs in vegetables as wells as meat. In the montage to the left, I soaked six, 2" diameter red skin potatoes in a 7% salt brine, following the same imaging procedure as with the pork slices. The top hemisphere was peeled to expose the potato flesh and remove the "cortex" layer just below the skin (thus the faceted edges). The cortex has a different cellular structure than the interior, and might distort the diffusion results. Interestingly, potato skin, at least in 32F water, is impermeable to salt, which is why no salt band appears on the bottom edge of each slice. Perhaps the waxy nature of the potato's skin (the periderm) is the cause. As you can see, the salt front slowly marches steadily in towards the potato center. Since the potato is in an ionic bath of fixed concentration, the concentration profile roughly follows a complex error function:
For a fixed concentration level (as indicated by the transition from white to red), the variables in the () must be also fixed, so
In the real world, the potato is also affected by it's long soak in brine- slightly browning and softening, and losing 5% of its weight a day. Clearly, other cellular compounds are entering the water bath (probably a combination of osmosis through cell walls, and diffusion via the now porous intercellular matrix. Both process can occur in parallel, depending on concentration). So the potato flesh no longer forms a uniform matrix for diffusion, as presumed by the error function equation. Still, the trajectory is roughly right, and the fit to a square root in time, tolerable:
Given the very slow absorption rates, its clear the idea of using a potato as a "sponge" to remove excess salt from a broth, is one more food myth... |
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |

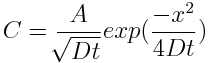


 A number of readers asked how quickly MSG (monosodium glutamate) diffuses, compared to salt. The MSG molecule is a bit larger than sodium or chlorine ions, but relatively compact, so one might expect it will diffuse more slowly, but still faster than sugar or many flavor molecules.
A number of readers asked how quickly MSG (monosodium glutamate) diffuses, compared to salt. The MSG molecule is a bit larger than sodium or chlorine ions, but relatively compact, so one might expect it will diffuse more slowly, but still faster than sugar or many flavor molecules.