| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| it's the salt, stupid | |||||||||||||||
|
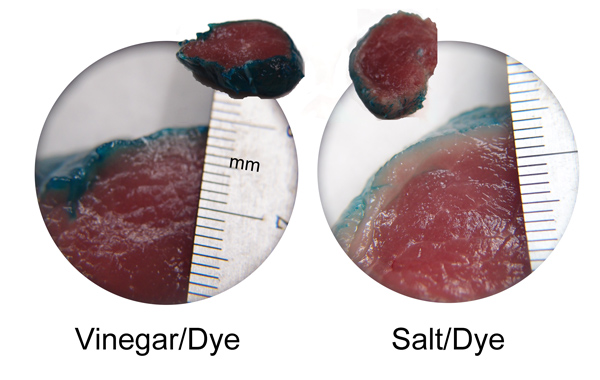
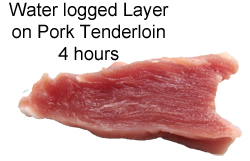
September 2011 Only a few cuts of meat are naturally moist and tender. And these few are often expensive and fatty. To utilize cheaper or drier cuts of meat, many cookbooks advocate salt-brining to add moisture, flavor and tenderness. Salt, at one time valued and traded like gold, has always been associated with food. In the absence of refrigeration, brining in salt water for days or weeks began as a method of food preservation. Other cultures dry-brined by rubbing salt onto food and waiting a few hours or days for it to penetrate. For many, salt spelled the difference between life and death. Today, salt brining plays a more limited role tenderizing or moisturizing dry cuts of meat just prior to cooking. While brining has many proponents, some people wonder if the entire process is a culinary myth. They've tried brining, and just don't notice much of a difference. Debates over brining constantly break out on web sites like amazing ribs or bbq-brethern, especially around Thanksgiving. Even world-class chefs (and their signature recipes) run the gamut from advocating overnight brining to simply salting the skin just before cooking. So let's test if these claims have merit. THE BIG BRINING THEORY The "theory" behind wet salt brining is a bit confusing, but claims to work it's magic via three steps. First, thanks to the wonders of osmotic pressure, brine diffuses into the meat, keeping it moist and tender. Second, it forms an impermeable surface layer that "seals in moisture", blocking fluids released from otherwise dry cuts during cooking. And third, salt chemically breaks apart meat proteins, tenderizing (along with flavoring) the dish. We'll test all three hypothesis in a series of experiments, varying the choice of meat, the brine concentration, and the type of brine. And learn a few cooking tricks along the way. Now, in addition to salt, other common brines (or marinades, which are basically thicker flavored brines which usually do not contain salt), includes acids (like lemon juice and vinegar), or bases (like baking soda). So we devised a test using all three classes, plus an unadulterated control (plain). To accentuate the brine's effect on the surface chemistry, in this first test small (3-4oz) pieces of meat (pork, turkey and chicken) were chosen to increase the surface-to-volume ratio, and thus the brine's influence. The brines were as concentrated as practical and the meat marinated for an hour in the fridge. The salt and baking soda brines were saturated solutions (e.g. mixed into water until no further chemicals are absorbed), or full strength kitchen vinegar (5% concentration, pH 2.5). Such levels may seem high, but are actually quite common in many traditional recipes from around the world. We weighed the meat before and after brining, measured the temperature rise of each sample during baking, and remeasured the final cooked weight. Plus tasted each sample, and checked systematically for tenderness with a simple indentation probe. If brines play a significant role, sealing the surface, small samples and strong brines would highlight its effect1. WATER, WATER EVERYWHERE BUT NOT A DROP TO DRINK Our first observation notes how little brine is actually absorbed by the meat- typically under 5% by weight in the salt brine, 2-3% in the vinegar and almost none in the case of baking soda. Plus, any absorption was limited to a thin surface layer, as revealed by this dye test: In the image below, pork tenderloin was placed in the brine solution for an hour, along with a strong blue dye. While the tiny salt ions were able to penetrate about 2 mm into the the pork (forming the paler meat layer just below the surface), the larger dye molecules remained in the brine. On the other hand, vinegar effectively unraveled surface muscle bundles (often referred to as "chemically cooking"), letting blue dye quickly and intensively saturate the immediate surface.
Geometrically, a thin brined surface layer is consistent with the observed few percent weight increase2. In the case of a more realistic three to ten pound piece of meat, the proportionately smaller surface-to-volume ratio limits absorbed water to well under a percent. And this is a well-trimmed cut- many people brine with skin, membrane, or a fat-cap in place- further restricting brine contact. In other words, the idea that direct brine absorption effectively incorporates significant amounts of water is a myth. This is why commercial meat producers use hundreds of tiny hypodermic needles and vacuum marinating to directly inject water into the meat's bulk. And they add gelling agents like food gums or functionalized proteins to bind water to the meat, so it doesn't drip back out during cooking. Simply put, brines just don't carry their own weight. SIGNED, SEALED AND DELIVERED But, perhaps other effects, like the rumored "chemically sealed surface layer" actually makes a difference. Here are four brined pork tenderloin samples photographed after baking at 235F until the internal temperature reached 175F (a bit above perfectly cooked, but frequently the end point for many home chefs). The plain and salt-brined pieces all lost around 20-25% of their initial weight, while the vinegar-brined meat lost 30% (we encountered similar results for chicken and turkey). All about equal tenderness, with a slight edge for the salt brine. 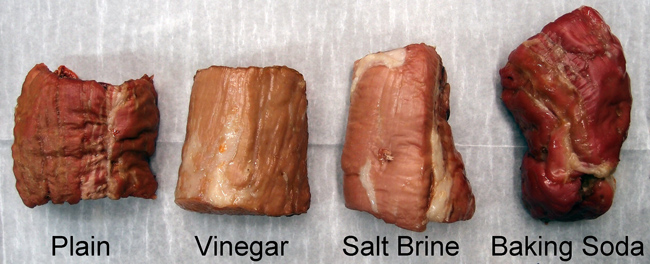
The greater loss with vinegar is expected after examining the temperature curve for each sample (while in the actual experiment all samples were cooked simultaneously to the "done" point and remove individually from the oven at 175F, I replotted them from the endpoint backwards in time, to allow clearer trend comparisons.) As you can see from this plot, the "plain" and salt-brined samples (blue and purple curves) behaved pretty similarly. But the vinegar curve (red) took much longer to cook, and more gradually approached "done". As we explained in the article on barbecue stall, slower rising temperature curves are the result of evaporative cooling. That is, water migrates to the meat's surface during cooking and cools the meat- just like sweat on a hot summer day.
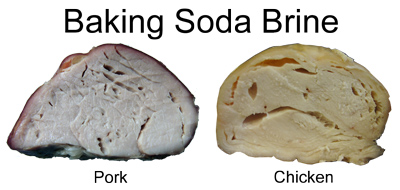
Since the vinegar-brined sample stayed cooler and took longer to cook, it must have evaporated a greater proportion of its initial weight. What about the baking soda brine, which cooked fastest? As you can see in the photo above the graph, a dark, plastic-like film forms over the meat. Tap it with a fork, and it sounds like a hollow milk carton. Talk about chemical sealing! Baking soda brines generally cook fastest because this tough sealing layer prevents evaporation. The meat also tasted a bit metallic. Unlike the other brine samples (which appeared smooth and moist in cross-section), the baking soda brined meats were filled with bubbles and voids, extending well below the thin tough surface layer.
In earlier experiments we demonstrated that brines primarily deposit chemicals on the meat's surface, which (depending on the chemical's form) quickly diffuse towards the center only after the temperature is raised. They do not diffuse very far inward during a few hours brining in the fridge, although it may seem that way based on tasting the final product. It appears baking soda unravels meat proteins on the surface quite effectively, where they polymerize into a tough skin during cooking. The baking soda also migrates rapidly into the bulk of the meat during cooking, forming gas bubbles like a leavening agent does during bread baking. At lower concentrations, baking soda brines combine mechanical with chemical tenderizing. In fact, salt and baking soda marinades are the basis of the appropriately named Chinese "velvet chicken" stir fry. Given these dramatic effects, a baking soda brine is best for thin slices of meat quickly marinated at low concentrations to avoid a metallic flavor. It's too easy to cross the line from tenderness to mushiness. ONLY IMPERMEABLE LAYERS LOCK IN MOISTURE However, we CAN treat and seal the meat's surface by drawing upon other common cooking ingredients. For example, by dredging the meat in dried egg white powder it forms an extremely sticky surface layer that hardens into a shell during baking. Xanthan gum powder yields a remarkably slimy layer with similar properties. Both food additives are commonly mixed in sausage or other ground meat products to retain moisture3. In the graph below, note how sealing the meat's surface causes it to cook more rapidly than untreated plain meat. It also loses between 10% and 15% of its original weight, rather than 20% (untreated) or 30% (vinegar brined). The meat is slightly moister than any of the brines, but note that moistness and tenderness are two different characteristics (I've observed plenty of fast-food chicken breasts oozing clear liquid, but are as rubbery as an eraser). The egg white seal is nearly as effective as wrapping the meat in aluminum foil- which completely halts evaporative cooling, allowing the enclosed meat to approach the boiling point of water: But most people brine in salt or acids, which we tested in a related experiment with turkey breasts. The plain and salt-brined samples cooked fastest (e.g. you could start cooking 45 minutes later, and still catch up with the vinegar sample), losing 20% of their initial weight. The vinegar-brined sample lost 30%, before reaching "done" (around 160F for turkey). I believe acids create a meat surface that does not easily reseal at high temperatures, allowing moisture to escape and flavors to penetrate. Which is why so many marinades are acidic... Given how similar unadulterated and the heavily salt-brined meat behave, its hard to imagine the existence of some special impermeable surface layer, on par with egg powder or baking soda, that makes much of a difference. Were such a layer to exist, by inhibiting evaporation it would result in less cooling and thus it would cook faster than the plain baseline. But no such faster cooking is observed. And practically speaking, any gelled layer is unlikely to be as smooth and homogeneous as saran wrap- the juices would find some way to blurt out. But, there is one last part of the brine story which does hold water. Literally. SALT = MOISTURE + TENDERNESS Perhaps salt does not form an impermeable baggie around the meat. Perhaps brining does not pump in water by osmosis. But food scientists know salt can "denature" meat proteins- that is break apart the bonds holding them together. Once broken, the denatured proteins, with the help of salt ions, are capable of attracting water electrostatically, even gelling or polymerizing with other broken bonds during cooking. To test this possibility, we measured the weight loss in lean 8 oz boneless pork chops. One sample was plain (e.g an unbrined control). The second sample was marinated in a standard 6% salt brine for 4 hours. And the third sample was dry brined. I rubbed on 1% salt by weight4, and let it sit in the fridge for 24 hours. Here are the weight loss results at two different temperatures:
Note they all cooked at about the same speed- no evidence of surface layer. But the brined samples were significantly moister. When I employed a salt revealing dye to image the distance salt penetrated during cooking, the 24 hour dry brine went in about 1.5 cm, and the 4 hour wet brine 1 cm (orange indicates the absence of salt).
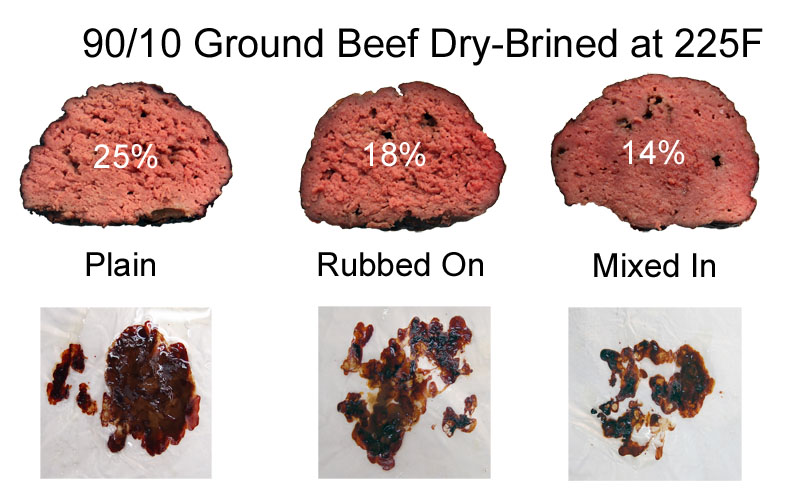
Comparing the relative proportions of salted meat, and assuming unsalted meat loses weight at the same rate as the control, this alone explains the difference between the two brines. More salted meat, less moisture loss. I also performed a number of "surgical tests". For example, I sliced a raw brined tenderloin in half, then reassembled with the brined surfaces facing each other on the inside, leaving unbrined meat on the exterior. So there was no way to form a gelled skin during cooking. But this "inside out" sample lost exactly the same amount of weight as an uncut brined sample with the salted side facing out. In other words, what matters is the total salted volume, not the salted surface area. Knowing it takes a full day for salt to diffuse 3/4", I dry salted a chicken breast over night at the 1% salt by weight level. Plenty of time for the salt to fully penetrate. Baked at 325F until the breast reached 160F. The salted breast lost 13% of its weight, compared to 20% with the unsalted control, and was much more tender. Since only around 40% of the meat contains evaporable liquid, salting preserves almost twice the juices of not salting. Its a significant effect. But for the most telling confirmation of the water-binding hypothesis I used ground meat instead of a solid cut. The large surface area of ground meat speeds up salt diffusion, and its possible to introduce salt uniformly throughout the meat's volume by simple mixing. Here we compared three 8oz (225gm, 3" diameter) balls of 90/10 ground meat. One sample, the plain control, was simply formed into a sphere. A second lump was formed into a sphere and sea salt (at the 1% by weight level) was shaken over the exterior. And finally I took 8 oz of loose ground beef, drizzled on 1% salt by weight, and then gently formed into a ball. After marinating for an hour in the fridge, they were carefully weighed, and then cooked in a 225F oven until each sphere reached 150F. As you can see from these cross-sections, the rubbed on salt (middle image) penetrates about 3/8", causing that surface layer to congeal, destroying the iconic, crumbly texture of ground beef. The internally-salted sample was converted entirely into a less porous, moist gel that is exactly unlike steak5. But definitely meaty (something to consider when you add salt, or salty liquids like soy sauce, to meatballs or meatloaf. When to salt matters.)
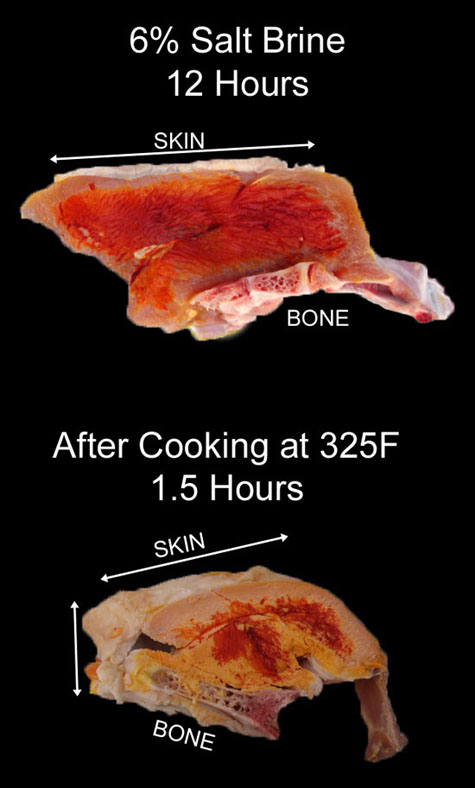
The numbers in white indicate weight loss (fat plus moisture) 15 minutes after cooking, while the square images are photographs of pan drippings captured under each ball. The 15 minute rest period is critical. While the "Rubbed-On" and "Mixed-In" weight losses measured immediately after cooking were essentially identical, when measured 15 minutes later, the "Rubbed-On" sample continued to ooze liquid from the unsalted interior. Almost a quarter of its total weight loss occurred during the 15 minute resting period (a similar phenomena was observed in pork)6. So, a thick salt-modified layer does SLIGHTLY hold back internal juices, but imperfectly so, and fluids will eventually squeeze out. Its the appearance of moisture, but the not the reality. Similarly, thinner pieces of meat can be rapidly cooked and remain moist, by heating faster than the juices ooze out. They will be tender and juicy for a while, though without the binding advantages of salt, often dry out by the next day. Salt-bound juices, on the other hand, holds onto moisture during cooking and during chewing. These moisture-preserving benefits depend on the final cooking temperature. At low temperatures, below 100F to 130F, muscle fibers have not yet unwound. So, in any case they would not squeeze out liquid, and salt's only role is as a tenderizer and flavor enhancer. However, above 130F juices begin oozing out robustly. More than 90% of all water is contained between the muscle fibers. Salt creates small electrostatic barriers, and these barriers help bind the water to the muscle matrix. We can measure this temperature dependent effect in a simple experiment on ground turkey. To eliminate the intertwined role of fat, I used 99% lean ground turkey breast. One 8 oz ball was hung by a string in a 225F oven, so I could weigh the meat during a four hour cooking run. A second 8 oz. sample was prepared by gently mixing in 1.5% salt, resting for an hour in the fridge, then forming into a ball, and baking. As you can see, around 110F the weight-loss rate accelerated (remember, when the interior is at 110F the surface is usually above 170F). But, the salt-brined sample (blue curve) lost weight much more slowly than the control (red curve). For example, at 150F, the plain ball lost 50% more water than the salt-brined sphere. Since white meat chicken, turkey and pork tenderloin are generally served between 135F and 160F, there is no question brining will prevent those dishes from prematurely drying out when cooked low and slow. SALT MARCHES ON Many people wonder if the advantages of soaking in a liquid brine are blocked by skin, bone or membrane. So we brined two, bone-in, skin-on 3 lb turkey breasts in a 6% salt solution for 12 hours7. After brining, we used the salt-imaging dyes to track the brine front. As you can see, the salt penetrated about 5-8 mm. A bit shallower near the bone or skin, but overall the brine penetrated uniformly from all directions.
After cooking in a 325F oven for around 1.5 hours until the interior reached 155F, we again sliced out a sample and imaged. Recalling that high salt concentrations (above 1%) are clear, medium concentrations (around 0.5%) are pale yellow, and no salt is deep orange, it's clear the salt front marched in from all directions. A 1" thick pork fat cap will block most brines. But turkey skin is only half fat, and in earlier experiments we demonstrated it can absorb both salt and water while limiting the diffusion of larger flavor molecules. Bone is riddled with microscopic tunnels to carry blood, oxygen and nerve fibers into the marrow. So it's not much of a barrier to salt ions. Plus turkey bones, like most birds, are very light and porous. Especially in young animals (and most commercially grown turkeys are processed before 20 weeks.) Bones impede, but not block the motion of salt ions. So wet brining is effective bones in or bones out. On the other hand, wet brining is limited in depth. Ion diffusion from a cold brine bath only penetrates about half an inch, while cooking merely distributes the salt by another half inch. Not much help with a 6" diameter, 10 lb pork shoulder or a 15 lb turkey. The solution is to inject a salt brine deep into the meat using a syringe. As a side benefit, the injected brine may contain herbs or other complex aromatics whose molecules are too large diffuse in from the surface. So flavor is carried along with salt. And, you can inject 5% or even 10% water, compared to the 1% typically absorbed by a large cut of meat from a wet brine. How well does injection work? Ah, there's the rub. Injection is quite variable compare to wet brining. If you inject too much brine creating a "pocket" or mis-align with the meat's "grain", it will squirt out before cooking. So it's hard to control the total amount of injected liquid, and thus the final salt content. Deep injection tends to leave the surface unbrined, so the surface is more prone to dry out. And, it's easy to miss a spot. So, when I brine I like to inject small amounts with a thin needle (0.05") in multiple locations, then wrap in saran to capture any exuded liquids to simultaneously surface brine the meat (turkey skin offers a similar effect as saran. Or you can apply a VERY lightly-salted dry rub. Or place in a wet brine). Wait 12 hours for the salt to do its work, then cook. Measurements on turkey, brisket, pork and chicken consistently demonstrates the injected meat is more flavorful and tender than either plain meat or meat injected with pure water. Flavor is delivered by the aromatics, and tenderness from the ability of salt to break apart muscle. This is true8 whether cooked at 225F low and slow, or fast and hot at 325F.
CONCLUSION So what do these tests imply?
|
|||||||||||||||
|
--------------------------------------------------------------------------------------------------------
1 We also tested weaker brines, where the effects on cooking time and moisture were proportionally diminished and less easily distinguished from the control. In pure water, the surface of the pork turns gray and mushy- after four hours, to a depth of around 2 mm. But no combination of chemicals and concentration levels ever raised the weight gain higher than a few percent. In a salt brine, the surface is pink and more rigid- osmotic pressure from salt penetration plumps up the cells. However, as we discuss in our article on diffusion, osmotic pressure inhibits brine penetration within a cell, giving the edge to salt ions diffusing through the juices and in the spaces between the cells. 2 The 3.5 oz. samples were about 4 cm cubes, and thus has a volume of 64 cc. The 2 mm surface layer has a volume of 4 cm x 4 cm x 6 sides x 0.2 mm=19 cc, but perhaps only 20% represents added water. So the percent weight gain would be 20% x 19 cc / 64 cc= 6%, in rough agreement with the observations. Most meats contain 60-70% water by weight, so 6% is a small contribution. And likely to ooze out quickly, because the brine is not chemically bound to the meat (e.g. known as "free" water). In a larger piece of meat- say 10" on a side, a similar calculation (presuming the surface layer remains 2 mm thick), yields a water weight gain of less than 1%. Underwhelming. Also, in a larger piece of meat, surface treatments have a smaller effect on the rate that water emerges from the interior- the internal moisture pressure overwhelms the surface resistance, until much of the moisture has evaporated. Surface-to-volume ratio dominates again. 3I sometimes sprinkle a small amount of egg white powder into ground meat when forming hamburger patties- they never fall apart and are definitely moister. 4Cured meats, like salami, typically contain 3% salt by weight. This is way too salty for steak. I prefer to dry brine meat at the 0.5% level, which corresponds to about 1/2 teaspoon of fine sea salt per pound of meat. Dust the salt on uniformly and let "marinate" overnight. For brining accuracy, I normally recommend the use of weight rather than volume measures, but for small amounts of salt, most kitchen scales offer too little resolution to be of much use. This is one time when volume wins. 5Below 2-3% salt, chloride ions gently denature meat proteins, electrostatically attracting and binding water to the meat fibers. Above this level, the severity of chemical bonds destruction is so high, loose protein segments rebond to each other in a disorganized manner, forming a gel. Wet brines exceed the 3% level only near the surface, which is also destabilized by localized osmotic pressure drainage- the first mm is already a sodden, gummy mess. Because diffusion is slow and ions have time to spread out, internal salt levels rarely rise above 1% in the bulk of the meat, and the gelled surface is incorporated into the crust. But, in dry brining the salt levels are near 100% around the salt crystals, and gelling is a more serious consequence for porous meats where this concentrated brine can be sucked into the open spaces like a sponge. In solid cuts of meat the salt combines with exuded meat juices forming a concentrated slurry, but again this slurry can only slowly diffuse inward. Still, one expects dry brined meat to brown differently then wet brined. On a more positive note, meat gelling is one reason you should consider lightly salting ground beef immediately before shaping and then cooking hamburgers. It helps keep the patties from breaking apart during grilling, and the small amount of water exuded is irrelevant. Don't form the burgers hours in advance, or add more than a quarter teaspoon per pound, unless you plan on serving dense hockey pucks on a toasted sesame bun. 6At 325F in 80/20 ground beef, the rubbed-on salt layer was less effective. On the one hand, fat is less affected by salt, and much of the weight loss was fat. Also, cooking times were nearly twice as fast as at 225F, so the salt only had time enough to diffuse in an 1/8". A thinner salt layer retains less moisture. Finally, the outside temperature of the meat sphere raced ahead of the interior, rendering out additional grease. So the Plain and Rubbed samples lost 30%, while the Mixed-in sample lost 20%. Still, brining made a difference. 7As common for most supermarket white meat turkey, the breasts were treated by the processor to "retain no more than 6% water", but had not been brined. The raw, as-received salt concentration was 0.1% or less. 8Final weight loss after injection is more variable than with wet brining, whereas wet brined meat almost always retains more mass than unbrined. There are a couple of reasons. If you don't wrap the meat with saran to brine the exterior, the surface will ooze juices during cooking (and a 1" thick surface layer might encompass a third of the meat's volume). While salt injection tends to swell muscles around the injection site, locking in small pockets of water, sometimes these pockets drain out in a burst during cooking. Also, the amount of retained juices depends on whether the meat stalls and on the final cooking temperature. For example, in this test we injected brisket with plain water, salt brine, or a gel mixed from xanthan gum and water. Each brisket weighed around one pound, and we added 6% water by weight. The salt brine (if no brine leaked out) would add 2% salt by weight, in addition to the water: Note how the salt and xanthan gum injections stalled for a longer period of time compared to plain meat or meat injected with the same amount of water. These treatments chemically bind with moisture, and thus the meat can evaporate for a longer period of time compared to plain or water injected brisket (which tend to dribble out streams of juices during cooking, wasting valuable liquid). Interestingly, once all four samples reaches 180F, they all lost 40% of their original weight. Only the salt injected brisket was tender and slightly more juicy. Slow and low cooking dries out meat, but compensates by offering enough time for muscle to break down and collagen to gelatinize. The added moisture from salt brining or xanthan gum aids with the gelatinization process, but eventually evaporates during the stall. For meats like turkey cooked to a lower temperature (e.g. 155F), the salt injected meats held onto 1/3rd or more juices compared to plain meat or plain water. The exact amount depending on how uniformly you injected, whether there was a water pan in the oven, air currents, etc.
|
|||||||||||||||
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |
|||||||||||||||