| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| dyeing to get in |
|
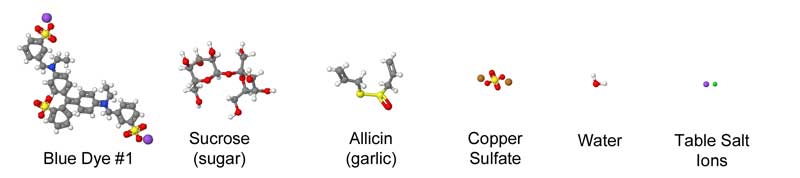
July 2012, expanded March 2014 Meat, at its most basic, is a protein and collagen sponge, storing up to 75% of its weight in water. Some of this water is bound chemically to the meat, while other molecules are "free" and can be squeezed out with a bit of pressure. Thus, when adding meat to a brine or a marinade, it's already fully saturated, and incapable of absorbing more than a few percent of additional liquid. Surprisingly, it sops up even less flavor. Which is curious, because so many recipes call for a long soak in a liquid bath so the meat won't dry out during cooking, but also to add flavor. Indeed, when heating a meat "sponge" on a grill or in the oven, water and other juices (primarily myoglobin and fats) quickly escape. Some of this moisture evaporates, cooling the meat. At higher temperatures, rivulets of liquid ooze out as the meat proteins shrink, wringing the sponge dry. Cook too long, and its like eating shoe leather. Chefs hope by immersing the meat in liquid (braising) or wrapping in foil, some of this moisture will reenter the sponge. We could learn a great deal about the effect of cooking and marinating on meat if there was an easy way to follow the juices in, and out. Unfortunately, these juices are either clear or leave no track behind as they travel through a forest of muscle fibers and capillaries. But, if we can't see the juices directly, perhaps we could visualize the path by adding a dye to the liquid that travels along with the stream. And one accessible to the kitchen scientist. The dye would have to be stable, so the color remains vibrant at cooking temperatures1. That's the easy part. The bigger problem is one of size- most dyes are large and complex molecules that get tangle up in the meat instead of tagging along with the much smaller water as it drains. Immerse a piece of meat in most dyes, and even after a day it has penetrated only a mm or two. When you eat a hunk of marinated meat, your tongue is fooled into thinking the entire bite is flavored, when its just tasting the surface. On the other hand, eat a thin piece of marinated meat, like Chinese stir fry where the meat is entirely surface, and its soaked in flavor. Don't believe me? Next time you smoke an 8 lb pork shoulder, cut out a plug from an inch below the bark. It tastes porky, but not very salty and nothing like the rub and glaze you labored over for hours.... After much experimentation, I discovered copper sulfate is a reasonable tracking dye. The color is blue, and easy to distinguish in red meat or white chicken breast. Its available at most hardware stores2. And, when gently heated in water, the color hardly changes, even near the boiling point. Most importantly, the molecule is tiny- only a few times large than water, and 10x smaller than food dyes. Now, the mobility of a dye in meat depends on its electrical charge and shape. It might chemically combine with the proteins and stick. But copper sulfate is reasonably mobile and inert. One test of mobility is diffusion through a simple solid, like gelatin:
We can roughly judge the size of a molecule by counting the number of atoms it contains. Blue food dye contains 87 atoms- water just 3. Of course, many marinades contain sugar (sucrose consists of 55 atoms), or garlic (of the hundreds of compounds in garlic, allicin is most prominent at 20 atoms). Copper sulfate contains just 6 atoms, and thus is more like water than sugar:
Chemists have determined the shape as well as the composition of these compounds- as you can see from this approximate comparison, the difference in size and configuration is striking:
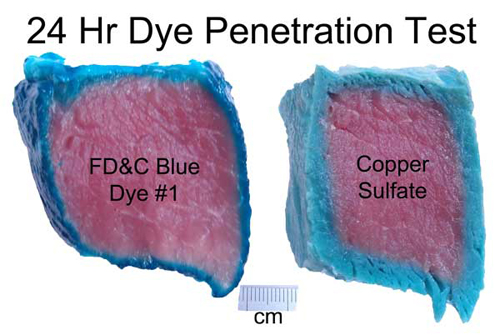
images from http://www.chemspider.com Now on to some kitchen science. As a first test, I marinated a hunk of pork tenderloin in a bath of water with blue food coloring, and one with copper sulfate. After 24 hours the food dye only penetrated a mm or two, while the copper sulfate diffused in as far as a cm in some places. And the mm is an over-estimate. The meat's surface is disturbed when cut with a knife- much of the dye penetrated only as a result of this torn musculature.
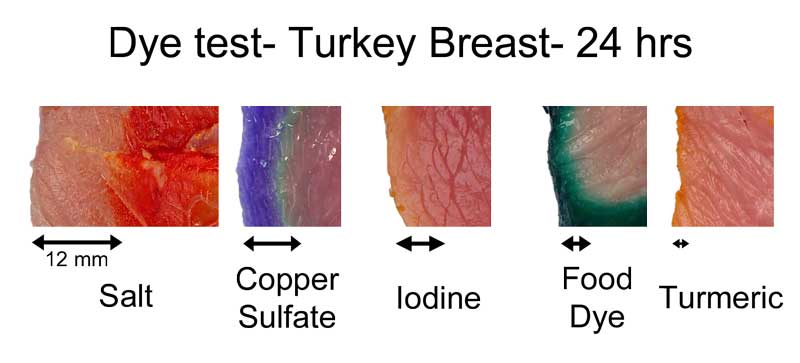
In earlier experiments, where we used a chemical "developer" to reveal the diffusion of table salt ions from a brine, these individual sodium and chloride atoms migrated about twice as far as the sulfate, in the same time period. How far depends on the meat, as well as the dye. In this montage3, I compared dye penetration in turkey breast after 24 hours in the fridge:
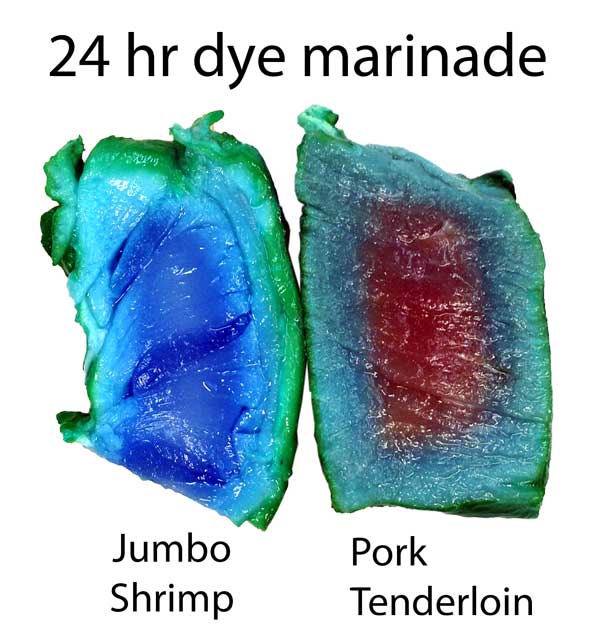
The copper sulfate in turkey diffused in about 2/3rd as far as in pork tenderloin. Turmeric, a common spice and colorant used in marinades, barely makes it past the surface. Meat may be a sponge, but it doesn't sop up all spills equally well. This is one reason a marinade may be too intense to use as a sauce- some components in a marinade must be "over-represented" to compensate for their poor absorption. In fact, slow diffusion is one reason4 why most salt brines are formulated at 6% while the ideal salt level (as a flavor component) is closer to 0.5%. Because salt may only accumulate in the top layer of the food, you need a high surface concentration to average out to a palatable bulk concentration when finally chewed or stewed. This filter effect is less of an issue for "open circulatory" system foods, like shrimps or insects. Meat, fowl, and squid distribute blood uniformly throughout a body via a dense network of veins, arteries, and capillaries. Blood never leaves this closed system. Instead, oxygen, glucose and waste products diffuse through the circulatory system's walls into adjacent muscles. Similarly, flavors have trouble working their way in from a marinade past this tightly constrained network. But, a shrimp's primitive heart simply dumps blood into open sacs (sinuses), where it washes over and around muscles and other organs. Plenty of voids and pores for marinades to enter. It's the difference between a drip irrigation network, and rain. Not surprisingly, dye diffuses much faster into shrimp than into meat. Here, I marinated a peeled jumbo shrimp and a pork tenderloin in a solution of copper sulfate and green food coloring. After twenty four hours in the fridge, the shrimp is colored throughout, while the pork still has a way to go. Also, notice the large green dye molecules penetrated farther in the shrimp than the pork, where they barely made it past the surface.
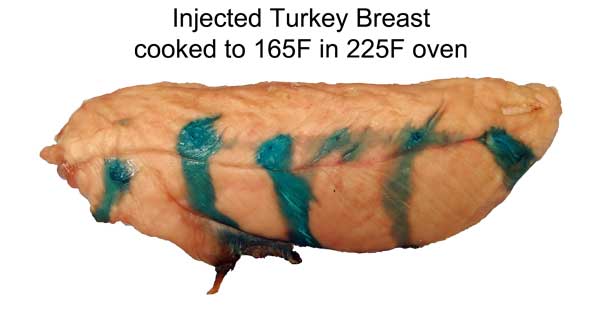
Cross-section after marination. Photo not retouched. Shrimp ~3/4" across. What does this imply for marinating? Well, an hour in a shrimp marinade is like a day for meat. So a shrimp marinade should contain much less salt, and will take less time to act. And, unlike meat, even complex molecules will flavor deep to the center of most crustaceans. So again the spice mixture must be adjusted accordingly. Don't use a meat marinade on shrimp! Initial juice experiments: Clearly, marinating from the outside-in works best for thin meat slices. To flavor a large cut of meat, direct action is called for- deep hypodermic injection. But exactly how effective is this injection? As an initial test, I injected a turkey breast in six spots, depositing a pea-sized glob of copper sulfate saturated water. Then I baked the breast at 225F until the inside temperature reached 165F. As you can see from this photo, much of the injected liquid oozed out onto the surface- the turkey looking more like a blue zebra than a beige cutlet. Some liquid is left behind- but as a rule, most of what you inject, ejects:
Cross-sectioning the breast is revealing, in more way than one. The breast meat, about 1" thick after cooking, begins to flake along the muscle groups. The dye travels down these cracks much faster than simply migrating outward along with the juices. Also, dye near the interior travels farther than dye near the exterior- clearly, the flow is mostly "outward"
Human eyes are excellent at detecting edges, but insensitive to smooth gradients. So, using Photoshop I saturated the red channel, more clearly delineating the blue copper sulfate
Fortunately, a bit of injected marinade does remain to flavor and salt the meat. In turkey breast, at least, you should inject every half inch to assure full coverage. Commercial injection systems employ a "bed of nails" arrangement of needles about this far apart- now you know why.
So what have we learned from these experiments?
|
|
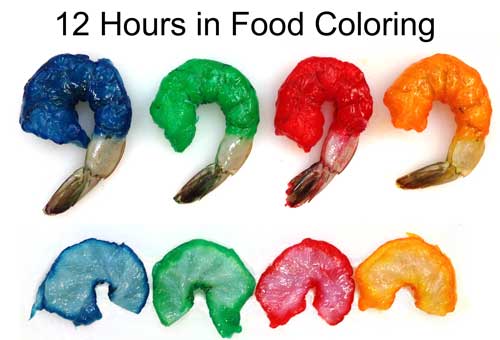
-------------------------------------------------------------------------------------------------------- 1Iodine is a great potential dye choice. The molecule is as small as salt ions and brightly colored. Available at any drug store. But, the color quickly fades at high temperatures, and while the red iodine color is easy to distinguish against chicken breast, its hard to track in red meat, or even uncooked turkey breast, as seen above. Standard food colors differ slightly in their absorption. These four raw jumbo shrimps were marinated in dye for 12 hours, then slit open. Note how the penetration distance varies by color. The center of the raw shrimp is actually nearly clear- but shrimp meat is translucent, so the surface color is visible through the meat.
2Copper sulfate is the sole ingredient in many products that clear roots from drain pipes, such as ZEP. While the chemical is mildly toxic to humans, it is deadly to fish, so for this reason many cities ban the addition of copper sulfate to the water system. If you choose to duplicate these experiments, please wear gloves and dispose of the waste materials safely and according to local regulations. Don't consume the meat! 3 In this photo, I used a pool saline detection solution to make salt visible. High concentrations of salt are clear, so you can see the meat. Low concentrations are reddish. I also increased the contrast of copper sulfate dye in Photoshop. 4 Food preservation is the other reason salt brines are so concentrated. But, if you brine for preservation (e.g. soaking salt cod or corned beef brisket in brine for weeks rather than overnight), the high levels of salt will densify the flesh, and must be washed out before consuming. |
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |