| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| setting limits | |||||||||||||||||||||
|
Jan 2017 Remember-- free advice is worth what you pay for. Use at your own risk! |
|||||||||||||||||||||
|
Preserving meat with nitrites is contentious, confusing and a potentially dangerous endeavor, muddled by a wide range of opinions and regulations. Never the less, we can cure safely and sanely, IF we understand how curing recommendations are set. And when they can be taken with a grain of salt.... The world is filled with naturally occurring chemicals that are necessary to life, yet potentially toxic, depending on dose. Too little salt, our nerves misfire. Too much salt, our heart stops and our kidneys shut down.
A related (but confusingly similar sounding) nitrogen oxide compound is nitrite NO2. NitrIte (note the "I" vs "A" and one less oxygen) is a bit less stable than nitrAte, and rarer. It is typically produced in acidic environments, or in lightning strikes, or more commonly, as a bacterial waste product.
Nitrites can be good. At least at "low" levels. Presumably, nitrites kills botulism more readily than it kills people- so it is thought of as a preservative rather than a poison. But how low is safe? Nitrites kill by blocking hemoglobin in red blood cells from absorbing oxygen- basically you suffocate at high nitrite levels. It turns myoglobin, which stores oxygen in muscle, pink. Curing your inside like a ham. And, it may form suspected carcinogenic compounds like nitrosamines in your stomach. Or accelerate nitrosamine levels when seared at high temperatures- like frying bacon in a pan. Determining safe exposure levels is a tricky subject. For one thing, it is unethical to systematically dose humans with chemicals until they keel over and die. Mice, perhaps, but no one is sure if mice expire in the same way as humans. Sometimes, an industrial accident or mislabeled food product inadvertently reveals the human toxic dose level. Fortunately, these are rare events. So toxicologists and epidemiologists "triangulate" on a safe dose. They start with the lethal dose as the upper limit. Since not everyone reacts to the same toxin in the same way, the accepted "Lethal Dose" threshold is LD50 where half the people die. Or, in the absence of accidental human poisoning, where half the lab rats perish. A few representative LD50s for adults consuming these substances are listed below:
Scientists then run studies searching for the LOAEL, or Lowest Observed Adverse Effect Level. These are non-fatal drug responses, like shortness of breath, or hives, or a diminished reaction time. While tolerable, these minor effects may telegraph the early stages of a more dangerous reaction, so are treated with respect. In the case of nitrItes, the LOAEL is around 1/40th the dose of LD50, or 5 mg/kg. And now comes the hard part: do you set the regulatory standard above or below the LOAEL, and by how much? You want to keep people safe, but also hope to minimize regulatory burdens on businesses. And leave people free to make their own mistakes. Clearly, a drink or two of alcohol is WAY above the LOAEL, yet legal. If it weren't for our long history and comfort with alcoholic beverages, we'd never tolerate the 10,000 deaths a year directly attributable to impaired LOAEL driving (can you imaging the public outrage if a food additive killed 10,000 people a year!). Not to mention the untold damage alcohol wreaks on so many families. Your freedom to poison your liver stops at your car's ignition... Children are more sensitive than adults to nitrite, perhaps by a factor of 10. So does a regulator set the bologna nitrite level at 25 ppm presuming adult consumption, or worry a child might eat a bologna sandwich every day for lunch and dinner, with creeping side-effects? Should we allow a parent to poison their child with a diet rich in lunch meat and salt in the name of "freedom"? There are no easy answers.
This threshold setting process may appear arbitrary (and in some sense it is), but doing nothing or simply claiming "my daddy smoked for his entire life and lived to 95" is hardly comforting. Drug trials and epidemiological studies are expensive and slow-- if we want to narrow the gap between caution and cancer, it will cost us. Before complaining about the inconvenience of regulations, put yourself in the shoes of the those bureaucrats whose decisions are truly life and death, and recall the old days of smoggy skies and arsenic laden water and millions of deaths a year from toxic exposure. The side-effect of a healthy public is a bit of unintended over-regulation. More often, the benefits are real, but the potential costs remain unknown. This is the case with nitrite curing. Only 25 ppm is necessary for color and flavor. We know a minimum 50 ppm level is necessary to avoid botulism growth and kill listeria. And people die from botulism. That is a clear benefit. On the other hand, there is weak emerging evidence that nitrites in the food system cause cancer or reduce lifespan or triggers some other disease. A few studies are worrisome, but the majority indicate there is no reason to be concerned. So regulation "threads the needle" and ends up pushing the standard as close to the benefit as possible. And as far from the possible danger- even if it is near the LOAEL. Because some of the nitrite will bond with meat myoglobin before it has a chance to kill bacteria, you might throw in a safety factor of 2-3. More or less, this is how we ended up with a ~150ppm nitrite level in cured meat. How large is 150 ppm in units of pastrami sandwiches? Well, if you eat a pound (500 gms) of cured meat, that contains 500*150/1,000,000=0.075 gms of nitrite. In a 150 lb person, the dose is 0.075gm/70kg, or 0.001gm/kg body weight. Which is 200 times lower than the LD50 of 0.2 gms/kg, but just around the LOAEL. Not much room to maneuver. In fact, most governmental agencies suggest limiting cured meat consumption to around 2 ozs a day, which brings the dose 10x below the LOAEL. A pastrami slider, not a belly buster. But wait, there's hope for the carnivore. The risk is actually lower than it seems. Why? Well, 24 hours after nitrIte curing, about half the added nitrite has chemically bonded with the meat as nitric oxide NO, and is no longer dangerous to consume. In a couple of days, only a few percent, known as the "residual level", may remain. This is why sausage makers, who leave meat exposed to air for weeks or years on end, add nitrAte to the meat. Benign bacteria1 in the meat slowly convert the nitrAte to nitrIte, ensuring constant protection against botulism bacteria from gaining a foothold days after stuffing the casing. The 150 ppm nitrIte regulation is the INITIAL dosing level to kill bacteria, not what remains in the meat at the deli counter. Much of the residual nitrIte also drips out during cooking. Or is sliced off with the fat cap when trimming a corned beef. In fact, a few percent of safe nitrAte in veggies is converted to nitrIte in your stomach and mouth2. Depending on your diet, the nitrIte load from veggies is much larger than that from cured meats. In many areas of the country, the water supply is highly contaminated with nitrAtes (mostly from fertilizer). At some point, attempts to lower your cured meat consumption are swamped by diminishing returns. A fact the regulations should take into account. Thus I'm comfortable eating a whole pastrami sandwich whenever the urge hits me. Which is pretty often. What does this all mean when curing at home? First of all, don't get freaked out if one recipe suggest 4 tsps of curing salts, and another 5 tsps. This difference is well within the scientific uncertainties. Second, use a cure calculator like this one right here at genuineideas. How it works is discussed in the footnote 2 below, but the Then balance that Italian sub with a nice fresh, nitrate-laden salad. Additional Reading
|
|||||||||||||||||||||
|
-------------------------------------------------------------------------------------------------------- 1 Because some customers are freaked out by the word "nitrite" on a package of bacon, a few manufacturers now claim their deli meat contains "no added nitrites, or is "all natural", or even "uncured". These assertions are almost always a lie. Instead of sodium nitrite, they start with celery juice, which is "natural" and contains high levels of nitrAte. You'll notice celery juice on the ingredient label. Then, after a round of bacterial munching or chemical processing (which is often NOT on the label), they convert the nitraAte into nitrIte. And cure as normal. The USDA requires the package label to say "uncured" because the actual nitrite and cure level is inconsistent. But I feel this is misleading, and prefer it would read "partly cured by nitrite from vegetable sources". Young children's immune system and digestive tracks are immature and may harbor bacteria that readily convert nitrAte to nitrIte. Commercial infant food manufacturers carefully monitor the nitrAte levels to avoid nitrIte poisoning. But occasionally a well-meaning parent cooks up a batch of homemade garden vegetables, and their child dies from methemoglobinemia. 2 Our brining calculator determines how much Prague Curing Powder #1 is safe and effective, based on the weight of the meat and the amount of brining liquid. Prague Powder #1 contains 6.25% sodium nitrite and the balance table salt. It is almost always dyed pink to distinguish from regular salt, but today there are pink "Himalayan" salts on the market, which are nitrate-free, so make sure not to get the two confused. The brining ppm calculation is a simple ratio of total liquids to Prague Powder, treating the meat as additional liquid, But, there are real-world effects which swamp the precision of this tool. For example, there are easily 10% swings measuring out a tablespoon of curing powder, depending on the spoon, the humidity and your technique. If the meat contains bone or thick fat deposits, you underestimate the final ppm concentration by simply inputing the total weight. The speed at which nitrite turns into inactive nitric oxide depends on the meat species, iron content, brining time, thickness, fridge temp and pH. Easily another factor of 2. The amount of bacteria present depends on whether you are brining a solid hunk of brisket, or sliced meat for jerky. Since, as this essay points out, higher levels of nitrite are unlikely to be dangerous except in extreme circumstances, we decided to keep it simple. An earlier version of this calculator compensated for fat and bone and pH. In this calculator, we just treat meat as more water, so the nitrite molecules distribute themselves evenly between the liquid brine and the watery meat. Close enough, exactly correct in many cases, and easier to understand. If you input "0" liquid brine, the calculator spits out the amount of curing salt required to dry brine. At 150ppm, this is 2 TBS of Prague Powder #1 for 25 lbs of meat. You can also select a nitrite level up to 200 ppm, and no lower than the 25 ppm level to color and flavor. (Note different guidelines apply to dry curing ground meats, for shelf-stable deli meats, etc.) There are three reasons Prague Powder #1 is sold as a mixture. First, with such tiny amounts of sodium nitrite required for curing, there is a high chance of a measurement error without the leverage of dilution in a bulk carrier. Secondly, salt brining adds flavor, helps retain moisture, and discourages bacterial growth. Thus the two ingredients are natural partners. The third reason is an early warning system. At 150 ppm of sodium nitrite, the salt level using Prague #1 is around a quarter of percent of the meat's weight. This is just right for serving. If by accident you doubled or tripled the nitrite dose (say by confusing tbs with tsps), the meat would be too salty to eat. Pay attention to your tongue! And your eyes- if the cured meat is normally pink after curing, but deep deep red this time, perhaps you overdid the Prague Powder. As to how long to brine, we've performed extensive experiments on nitrite diffusion in meat. The penetration time follows a simple thickness-squared law. By calculation and by experiment, a cylinder (like a pork tenderloin or a ham leg), cures about twice as fast as a flat for the same thickness. This is because in a cylinder, nitrIte can diffuse in from four directions, instead of two like a flat plate. Note the calculator is set to offer the MINIMUM cure time, which brings the center of the meat up to to 80% of the brine level. If you kept your work surface clean so other bacteria won't grow in the brine, and don't mind a little drainage of meat juice into the brine, you can often brine a few days longer with no adverse effects. 3 Given all the uncertainties setting curing levels (e.g. LD50 is an average death threshold based mostly on projecting rat deaths to humans, and the amount of nitrite required to kill botulinum depends on the species, pH and canning methods), why are the regulations so precise? Did it really make sense to reduce the nitrite level from say 200 ppm to 156 ppm? Directly, no. Indirectly, the constant, slight reductions are a "signal" to the industry to keep innovating. Lower is presumed better, because we are not sure how dangerous nitrite is, and because there are many alternatives to cured meats. In fact, if you read through the EU and US nitrite regulations, they are highly complex and nuanced and vary depending on how the meat is cured, packaged and shipped. They often argue over setting LOAEL safety bands, compromising on 500x reduction instead of 100x, in response to a consensus view of the latest studies. Again, the differences many be hard to justify by actual public health benefits, at least in the short term. But lower levels may be wise in retrospect.
|
|||||||||||||||||||||
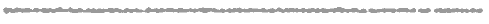 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |
|||||||||||||||||||||
 Nitrogen compounds are also essential to life (think nitrogen fertilizers or DNA) but are sometimes dangerous (as in nitrogen explosives like TNT or caustics like ammonia NH3). Nitrogen atoms are so reactive, they are almost always present in nature as either the inert diatomic molecule N2 (air is 80% N2) or oxidized as the nitrate compound NO3. This nitrAte is reasonably stable and safe, occurring in fruits and vegetables up to the 0.2% level. It may be an essential nutrient.
Nitrogen compounds are also essential to life (think nitrogen fertilizers or DNA) but are sometimes dangerous (as in nitrogen explosives like TNT or caustics like ammonia NH3). Nitrogen atoms are so reactive, they are almost always present in nature as either the inert diatomic molecule N2 (air is 80% N2) or oxidized as the nitrate compound NO3. This nitrAte is reasonably stable and safe, occurring in fruits and vegetables up to the 0.2% level. It may be an essential nutrient.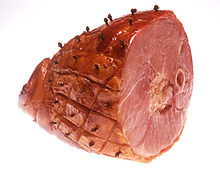 For centuries,
For centuries,  Most commonly, a wide safety factor below the LOAEL is chosen, and here science gives way to pragmatism and caution. Often a "round number" safety factor of 10x or 100x is proposed, depending on past history and a balance of cost-benefits. For example, when regulating a new substance (similar to others known to cause cancer and one which has few positive health or commercial benefits), it might be set 100x below LOAEL. This is a conservative choice, balancing the few anticipated benefits against an unknown but vaguely familiar risk. The safety factor may also insure against the negative effects that might arise decades later from low level, daily exposure. These low-level risks are very hard to detect in one or two year studies, but have popped up over and over again in the real world. With devastating effect.
Most commonly, a wide safety factor below the LOAEL is chosen, and here science gives way to pragmatism and caution. Often a "round number" safety factor of 10x or 100x is proposed, depending on past history and a balance of cost-benefits. For example, when regulating a new substance (similar to others known to cause cancer and one which has few positive health or commercial benefits), it might be set 100x below LOAEL. This is a conservative choice, balancing the few anticipated benefits against an unknown but vaguely familiar risk. The safety factor may also insure against the negative effects that might arise decades later from low level, daily exposure. These low-level risks are very hard to detect in one or two year studies, but have popped up over and over again in the real world. With devastating effect. dosing levels are close to USDA recommendations3. Keep your food prep area clean (there are other nasties besides c. botulinum lurking in the kitchen), make sure your ingredients are fresh and the curing salt has the concentration required.
dosing levels are close to USDA recommendations3. Keep your food prep area clean (there are other nasties besides c. botulinum lurking in the kitchen), make sure your ingredients are fresh and the curing salt has the concentration required.