| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| taking the cure | ||
|
||
|
May 2014 Summary of curing salt history and use:
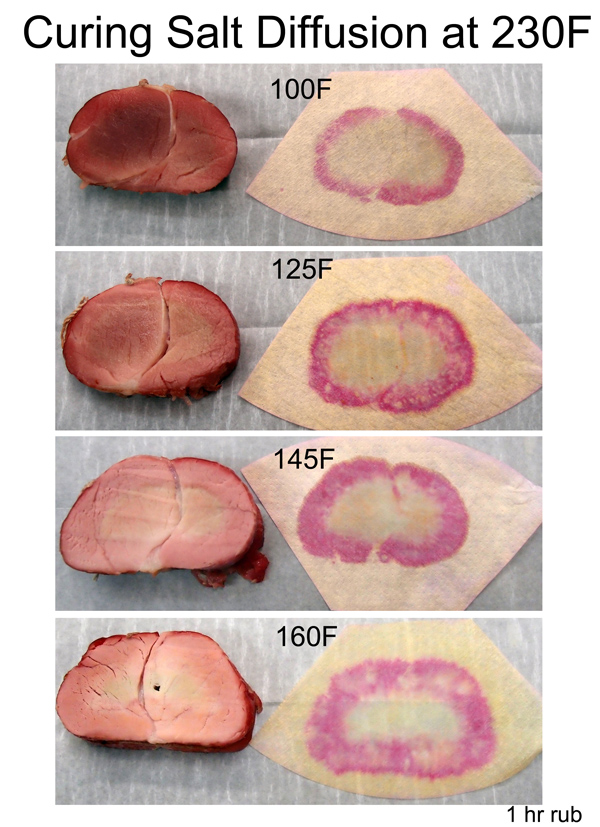
Food-on-demand is a modern luxury, and without a supermarket, life's a bitch. So once early humans were lucky enough to fill their stomach, thoughts quickly turned towards ways to preserve the excess. And soon developed a range of solutions. Drying food kills many bacteria outright by dessication, or by concentrating ions which destroy cell walls. Controlled rotting (e.g. fermentation) replaces bad bacteria with faster multiplying good, while the good bacteria's waste products often disable the interlopers. Salting dries out food and causes cell walls to burst by osmotic pressure- a two-for-one act. And salt is the true beginning of our story. Supermarket table salt is almost pure sodium chloride, washed and cleaned to avoid reminding consumers it was once churning seawater in which fish ate and peed. Seawater contains hundreds of chemicals along with bits and pieces of organic floatsom. Very commonly, the potassium and nitrogen from algae or bird poop, when concentrated in an evaporating salt water puddle, turned into "salt peter", or potassium nitrate (KNO3) and sodium nitrate (NaNO3). Migratory bird species often nest on the same islands for thousands of years, building up easily mined, thick deposits of guano. The major use of nitrates from bird poop were fertilizer (much more efficient than plowing manure or seaweed into a field) and as the strategic ingredient in gunpowder. Hard to imagine a resource more important than "guns and butter", so it's not surprising control of primarily South American guano bird poop islands led to war, slavery, boom and bust. In World War I Germany lacked access to New World guano deposits, but developed a way (the Haber process) to convert fossil fuels into ammonia and nitrates. This marked the end of renewable fertilizers, and today our low-cost food infrastructure is mooching off a three hundred million year old trust fund of buried oil and gas. Early on it was noticed that sausages preserved with salt or packed in ashes sometimes took on a pink coloration, even after cooking. It took many years to realize a complex series of reactions (including bacteria converting potassium or sodium nitrate to nitrite and acids converting nitrite to nitric oxide, which then colored and flavored meat and killed bacteria- phew) was at work. Nitrates occur in very high levels in some fruits and vegetables, such as spinach (up to 2000 ppm). These levels are often higher than the legal limits for curing meat with manufactured nitrates. In fact, many pink cured deli meats that advertise "no added nitrates" are actually preserved with dried celery powder- check the USDA food label for actual nitrate levels. High organic nitrate levels are one reason preserving food in wood ashes can be so effective, and why braised meat sometimes remains pink. Chemically preserved meat also allows time for complex flavors to develop. Air dried sausage or hams are a heady mixture of flavors from bacterial decomposition, oxidation and blooming spices. None of which would be possible if the meat rotted before curing. Nitrates are essential for human health1, and despite early concerns, after many large scale studies the connection between nitrate consumption and cancer or mutagnesis remains elusive. However, high levels of nitrites are toxic, and thus some regulatory attention is justified. Nitrites (NO2) are less common than nitrates (NO3) in nature because over days and months they are converted to the more stable nitrate form. It is this lack of chemical stability that makes nitrites so valuable. NO2 breaks down into NO and oxygen. The NO turns the myoglobin in meat pink. Nitrites also kills bacteria like botulism, which thrive in delicious low oxygen environments such as the inside of a salami. Today, sodium nitrate and nitrite are the most popular meat curing chemicals. Very low levels of nitrites are effective- just a few hundred parts per million. So they are often diluted with table salt to simplify application. The most common pre-mixed salt products are known as Prague Powder #1 (6.25% Sodium Nitrite) for cooked meats, and Prague Powder #2 (6.25% Sodium Nitrite and 1-4% Sodium Nitrate) for dry cured. They are often tinted pink to prevent confusion with regular table salt. "Pink salt", "Tenderquick" and "Instacure" are either synonyms or slight variations on Prague Powder. Current USDA regulations limit their application to 1 oz/100 lbs of meat, or 156 ppm (although this varies by the type of meat and preservation method). Only ~10-25 ppm is necessary to create a light pink coloration, ~120 ppm to kill the most aggressive bacteria. Heat breaks down sodium nitrite, releasing the nitric oxide molecule which does all its work before the meat cools down and potentially could spoil. But a sausage maker might hang their links for years without cooking. They mix Prague Powder #2 into their ground meat. The idea is nitrite provides an immediate burst of NO, while bacteria breaks nitrate down into nitrite over months, continuing to protect the sausage as it ages. High acid levels speed up the conversion of nitrite to NO, with the added benefit of killing salmonella and reducing the danger that nitrites in cooked meats are converted to possibly carcinogenic nitrosamines. The acid also reduces the ability of meat to hold water ionically, so the meat dries faster. In this series of photos, I show a pork tenderloin that was rubbed with Prague Powder #1 at the USDA limits. Then cooked in a 230F oven. Periodically, I sliced off a hunk of meat, and pressed the slab against a nitrite detection filter paper. Even at these minuscule levels, the tiny NO molecule is very mobile, moving almost as quickly as salt through meat. The nitrite's inward movement is clearly visible, and related to the formation of the smoke ring.
Clearly, by the time the meat is cooked, the nitrite will not have had sufficient time to penetrate all the way to the interior. You can take advantage of this slow diffusion rate to create a decorative pattern of stripes or polka dots, but most people want their bacon or ham to be uniformly in the pink. So, instead of leaving the rub on for just an hour, it is more typical to apply the powder for a minimum of two days, and often for as long as two weeks. How best to apply the powder? Aye, there's the rub. And the brine. Typically, in a brine you mix Prague Powder #1 with water and immerse the meat for a prescribed time. If the brine is at 156 ppm of nitrite, and you wait long enough, and there is much more brine than meat, eventually the inside of the meat will come into equilibrium with the brine. And be uniformly cured. This method is slow, but has the advantage of infiltrating every nook and cranny with liquid. If one side of the meat is covered with thick layer of fat, (which slightly slows down nitrite diffusion), given enough time the nitrite will enter from the trimmed side and so fully penetrate. The meat will also absorb some water from the brine- up to around 10%. This added water helps keep food moist when cooked rapidly (so it has no time to leak out- think a chicken cutlet on the grill). But it makes no difference cooking low and slow for hours. Some people, in a hurry, double or triple the brine concentration, and then pull the meat out of the liquid after a few hours. The top cm or so is now highly infused with nitrite, so they rely on increased diffusion rates during cooking to even out the concentration. In principle this method works, in practice it is not reproducible from animal to animal, and from day to day. Dry brining is an alternative to wet. In dry brining you weigh the meat, and then portion out the Prague Powder at USDA limits. Then, sprinkle the powder on as uniformly as possible. I like to sprinkle on no more than 1/8th the total amount on one side, flip and sprinkle another 1/8th, and repeat, to increase uniformity. The best way to sprinkle the powder is with a spoon- tap the handle of the spoon and small amounts of the salt will bounce out and onto the meat. Then leave in the fridge for a few days until the concentration gradients even out. Dry brining is precise and less messy and my preferred technique. I find wet brining often sogs out the meat's surface and interferes with bark formation. However, I first premix Prague Powder #1 with two parts fine table salt, and keep the mixture in a tightly closed container. Then dose meat at the 1/2 tsp./ lb level. Why this combination? Well, it results in meat nitrited at 140 ppm- safe, pink and still effective. It also salts meat at the 0.5% level, which is high enough to retain moisture during cooking, and to my taste, perfectly salty. My herb rubs never contain salt. This way I can add additional layers of spice and sugar at any time during the cook, without worrying the meat or its jus will inadvertently become inedibly salty. You can always sprinkle a bit of finishing salt on just before serving to brighten the flavor profile. If refrigeration were widely available, I doubt chemical food preservation would be as advanced or as diverse. We owe our tubular meat traditions to the lack of cold storage. And to poop-filled salt.
|
||
|
1Nitrates, which are found in high quantities in many vegetables, are rarely toxic to adults. But, bacteria can convert the nitrates to nitrites, which are more concerning. Ruminates, like cows, have stomachs filled with bacteria and often suffer from nitrate-nitrite distress. Some infants are born with immature digestive systems which allow bacteria to grow. Their enzyme system is also immature, and unable to repair the damage from nitrites in the blood and will turn blue (methemoglobinemia). An extremely rare disease. Never the less, the first six months of baby food are designed to be nitrate poor.
|
||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |