| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| the smoke lab | ||
|
||
|
May 2014 While it's fun playing with smoke and fire, a systematic exploration of smoke ring chemistry requires special apparatus and precautions. Thus it was necessary to move beyond the complexity of actual smoke, to the simplicity of the lab and periodic table. The results of these experiments can be found in the tabs above- this article is about the apparatus and experimental method. We're experts, don't try this at home. This is a DANGEROUS experiment. Nitric Oxide (NO) is nearly as flammable as oxygen. So there is an explosion hazard. In small concentrations, NO is merely an irritant with a mildly sweet odor, but at high concentrations it can burn away the inside of your lungs. Carbon Monoxide (CO) kills by displacing oxygen from hemoglobin. The affinity of hemoglobin for CO is 200x larger than for oxygen- so you basically suffocate in your own blood. Around 400 people die each year from carbon monoxide poisoning, and tens of thousands end up in the hospital, only to leave with chronic neurological health problems. To top things off, nitric acid will eat away at your skin or blind your eyes. Plus the experiment is expensive. Not to mention the apparatus looks like something out of a Hollywood B-movie action film. There is always a chance a passerby imagines themselves in the next installment of "24", and calls in Homeland Security out of an excess of caution and paranoia....
We're experts, don't try this at home. For safety,
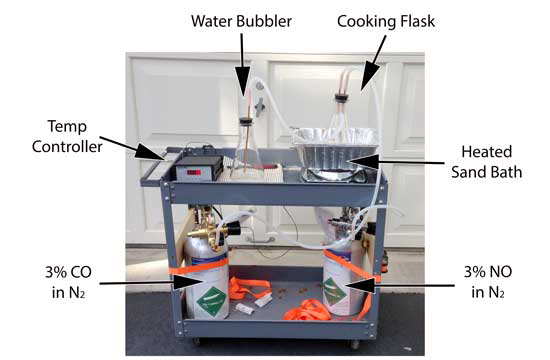
In the experiment, small rectangles of meat (typically 1"x1"x2") were suspended on a string in a stoppered flask buried in the heated sand bath. This is our "oven". The stopper admitted gas hoses from either the CO or NO tanks, down copper tubes to the bottom of the flask. Exhaust gases (CO, NO, water vapor and browned drippings) passed through another hose at the top of the vial into a water-bubbler flask. This bubbler scavenged some of the chemicals, but more importantly, offered a visual measure of the flow rate. The experiment itself was simple. After suspending the meat sample in the flask and inserting the stopper, one or both gases were bled through the flask. A bubble rate of one bubble a second was typical. With the stopper in-place, juices eventually raised the humidity level until the flask was covered with condensation. So we also ran a few experiments with the stopper removed, to lower the humidity. We also performed experiments with CO, NO and air in tandem, to determine if oxygen affected the ring. Finally we periodically ran contamination control samples , and confirmed there was no ring without NO or CO exposure. The meat was removed when its internal temperature reached 175F, assuring myoglobin had irreversibly broken down and would no longer turn pink in the presences of reactive gases.
On occasion, we also generated CO gas chemically via the well-known formic and sulfuric acid pathway. The acidic air simulates the acidity of most wood fires. This technique is also highly dangerous. Did I mention, don't try this at home! We also produced low acidity carbon monoxide by catalysis on charcoal. No acidity, and the CO concentrations were similar to those in smoke. The apparatus is simple- a stainless steel mesh bag (for smoking wood chips) is filled with activated charcoal (from a pet store), and placed on a propane grill side burner. The propane flame exhaust is mostly water and carbon dioxide. The CO2 flows through the very hot activate charcoal bed, and is converted to carbon monoxide. The rising carbon monoxide is captured by the aluminum foil tray containing the meat sample- the bottom of the tray was removed, allowing easy gas access: 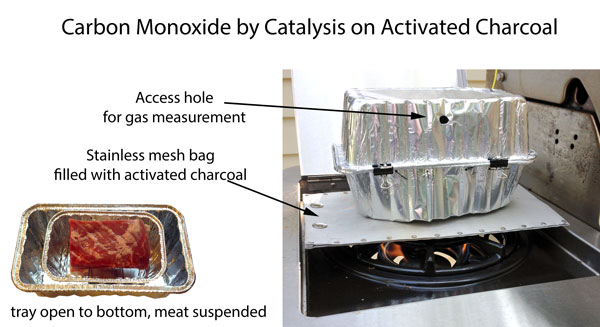
(Temperature ~ 250F. CO levels>10,000 ppm. O2 levels ~ 16%. NO levels <2 ppm CO/CO2 ratio ~0.2 Without the activated charcoal, CO levels ~250 ppm due to interference with clean burning conditions)
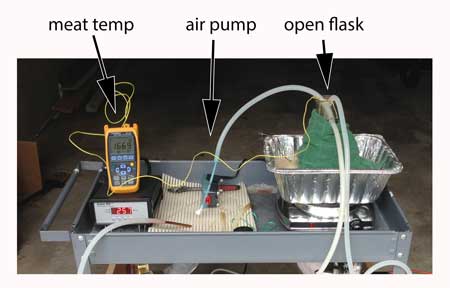
For the nitric acid tests, the meat was brined in 5 molar acid for times ranging from 15 minutes to overnight. Gloves, safety goggles, ventilation and drip trays were standard operating procedure. After brining, the surface was patted off and the meat heated in an electric oven until the internal temperature was 175F. A carbon monoxide "sniffer" was assembled from 3" PVC pipe joints and a 1/2" diameter aluminum tube. A battery-operated fan draws air through a HEPA filter (scavenged from a shopvac bag, and necessary to prevent smoke from contaminating the CO monitor), to a large rear chamber. The back of the chamber was sealed with a plastic sheet with a slit- once the CO level was established, a commercial hand-held CO monitor is slid through the gap into the chamber. Humidity or temperatures sensors can be inserted through holes in the side of the chamber. The sniffer cost under $15 to fabricate.
More information and results can be found in the tabs above, but here is some representative data: 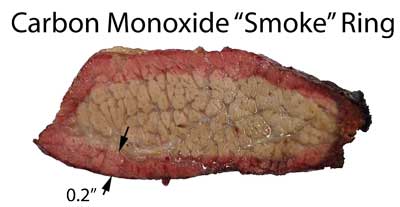
Brisket sample exposed to 3% CO/ balance N2 in open top flask for 3 hrs at 225F.
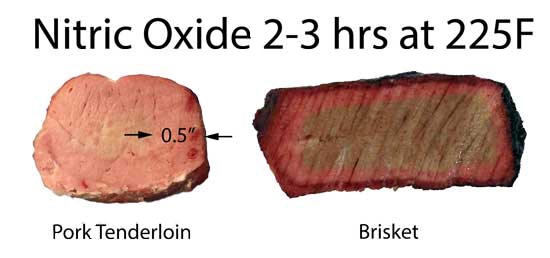
Pork and Brisket exposed to 3% NO and air in closed humid flask
|
||
|
|
||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |