| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| meet myoglobin | ||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
May 2014 Summary- meat color, including the smoke ring, is dependent on myoglobin:
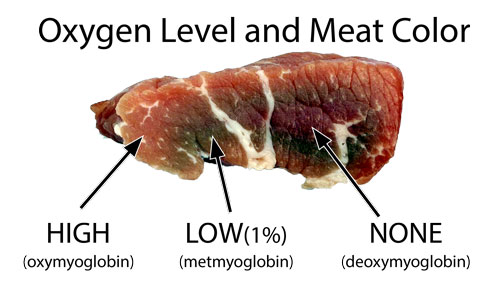
Whether scavenged, foraged, fished or hunted, humans have eaten meat for millions of years. A dense source of protein, fats and vitamins, meat is a convenient though not essential part of modern human diets. Cooking enhances the flavor of raw meat by converting this chemical stew into a heady mix of caramel notes (from meat's sugars), aromatic browning (the Maillard reaction between proteins and sugars, characterizing fresh bread or a singed steak), oxidized fats, and hundreds of lesser known, but welcome, compounds. All refined out a few dozen elements drawn from the periodic table. Cooking also softens meat, improving its caloric and vitamin uptake while killing off dangerous pathogens. Fire and meat- it's a good thing. In this "Atlas of the Smoke Ring", we are mostly concerned with how fire affects meat's color- particularly the smoke ring. And that requires a knowledge of myoglobin. When an animal is properly butchered, all the blood is drained away. So any juices emerging from a pink burger during cooking are water and myoglobin, not bloody hemoglobin. Muscles burn ATP (hmm, high school bio) with oxygen, just like a fire combusts wood with air. Except at lower temperatures, with no visible flames. Moving oxygen through the body to be "burned" by individual muscles cells requires two special couriers. Hemoglobin in red blood cells weakly grabs up to four oxygen molecules from the lungs, and carries them to muscle cells. At the cell wall, if the oxygen levels are low, the oxygen molecules jostle loose and pass through the cell membrane. Inside the cell, myoglobin (a molecule similar to hemoglobin which stores just one oxygen molecule tightly), holds onto the oxygen until demanded by the cell. Just like we control a smoker's temperature by adjusting fuel levels and air flow, the human body maintains a fixed chemical environment by finely tweaking its metabolism. Myoglobin also plays an important role regulating nitric oxide levels which are used to transmit biological signals. Nature is cheap and efficient, relying on myoglobin to mediate hundreds of regulatory processes. Scientists are discovering new pathways every year. The "glue" holding oxygen in myoglobin is an iron compound "heme". Iron rusts in air or water, and it's no coincidence that raw meat is rust-red in color. In fact, just like a corroded piece of iron, there are many colors of rust. And so there are with myoglobin. In raw meat, myoglobin adopts three colors depending on the extent of oxygenation. In high oxygen environments, like air on the surface of freshly cut meat, myoglobin is bright red. In zero oxygen environments, e.g. deep inside a steak, myoglobin is purplish. And in low oxygen environments (around 1%, for example in week-old packaged meats just below the surface), myoglobin is brown:
As you might imagine, this color change is reversible because myoglobin is DESIGNED to continually shuttle oxygen back and forth through the cell wall. So a raw gray meat surface is reversible to pink on exposure to air. Various enzymes speed up or slow down these reactions, and even in dead muscle, the enzymes remain active up to 150F, where their effect begins to taper off. Think of your brisket as "zombie meat", partly alive, partly dead, but still carrying on valiantly as if nothing had happened.... While a meat band may appear to the eye as a single fixed color, individual myoglobin molecules are constantly jumping between all three states. But local oxygen levels determine which state is more prevalent, on average. At fridge temps, oxygen moves slowly through meat- it takes perhaps a fews days for the brown LOW oxygen ring to appear in freshly cut, refrigerated meat. And a few minutes to a few hours for the surface to "pink up" when newly exposed to air1. Ninety percent of meat's color is due to myoglobin, but exactly how much myoglobin any individual cut of meat contains varies. Significantly. Active muscles, like dark meat, have 3-5x more myoglobin than white meat. Older animals may contain 5-10x more than young (think pale veal). But even within one species and one butchered animal, myoglobin levels and stability can vary due to stress, acidity, salinity, chilling rates, activity, pH, feed, enzymes, etc. As meat is cooked, the myoglobin molecule begins to irreversibly fall apart in the heat ("denature"). Depending on all the above factors, denaturing will take place around 160F (145F-175F) in beef, and perhaps 100F in tuna. Once denatured, it will no longer change color, even after exposure to high gas concentrations. Since heat penetrates food from the outside in, myoglobin progressively turns gray from the surface to the interior during cooking. A pattern familiar to any steak eater2. But iron can bond with molecules other than oxygen, and two of these molecules (carbon monoxide "CO" and nitric oxide "NO") are present in smoke. For example, carbon monoxide is 50x more strongly bonded to myoglobin than oxygen. Raw meat, exposed to CO and sealed away in a fridge, can maintain its bright, almost neon-pink color for over a year! The carbon monoxide form of myoglobin (carboxymyoglobin) is "metastable", and will slowly revert to myoglobin in a high oxygen environment. It reverts even faster when warm- such as during smoking or grilling (for more details, see the article on carbon monoxide smoke rings). Nitric oxide (NO) bonds even more tenaciously and rapidly than carbon monoxide to the iron rings in myoglobin, and will preserve the red color well beyond the boiling point of water. It prefers to bond with deoxymyoglobin to create nitrosomyoglobin, and will do so whenever myoglobin fluctuates into this configuration. Nitrosomyoglobin is a bright red compound and is pretty stable, but will degrade over hours in oxygen back to myoglobin. However, if the neon-pink nitrosomyoglobin is heated above the denaturing temperature (typically 145F to 175F), it forms a new, highly stable compound nitrosylhemochromogen (hey, sorry I didn't pick these multi-syllabic names), or one of a family of denatured hemochromes. And that is the darker pink color of cooked and some cured meats (for more details, see the article on nitric oxide smoke rings). Even this compound can turn gray-green when exposed to bright lights or air- but so slowly, you will have polished off the ribs long before it has a chance to fade. The top six rows in this chart detail these important color changes. The last three rows are included for completeness- myoglobin also bonds with a number of chemicals that are poisonous, and have no real purpose in cooking. But sometimes end up in our food.
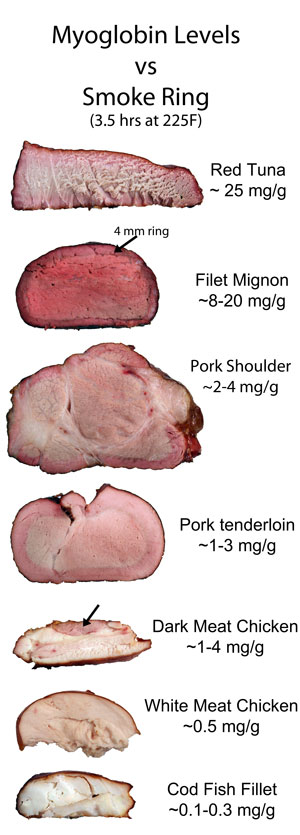
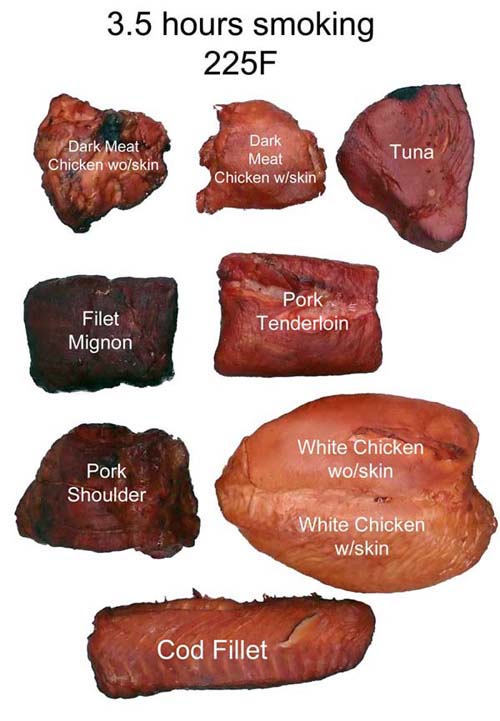
Since CO/NO preserves myoglobin's intrinsic color, it would make sense if the smoke ring (and even the bark color) might appear in proportion to the meat's myoglobin content. And you would be right. Here, we smoked 8 different kinds of meat for 3.5 hours. In cross-section, the correlation between myoglobin content and color is clear (click image for higher resolution file). And myoglobin also contributes to the bark's color:
Myoglobin, a molecule essential for life, is as fragile as life itself. It can "breath in" gases, and react by changing color. It can die when it overheats. And like every individual, it has its own, unique personality. Understanding myoglobin is a key to understanding how meat cooks, looks and tastes.
|
||||||||||||||||||||||||||||||||||||||||||||
|
1 Sometimes, particularly in ground meat where air can penetrate between the meat strands, the entire center contains brown, low oxygen "metmyoglobin". When you cook a burger medium well done (140-145F), remove from the grill and cut open, the center appears gray. But a minute later it turns pink, as oxygen and enzymes "reverts" the metmyoglobin to oxymyoglobin. 2 It turns out the surface pink myoglobin (oxymyoglobin) is more temperature sensitive than the inner purple myoglobin (deoxymyoglobin). So it denatures and turns gray at a lower temperature. This is one additional reason (beside temperature gradients) that a steak is encircled by a gray band. Some of that gray meat may be at the identical temperature as the inner pink, but its myoglobin was less stable. Tastes the same, just doesn't look the same. Another argument to rely on a thermometer to determine doneness. |
||||||||||||||||||||||||||||||||||||||||||||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |
||||||||||||||||||||||||||||||||||||||||||||