| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| carbon monoxide smoke ring | ||
|
||
|
May 2014 Summary carbon monoxide smoke ring
A pink smoke ring is a pitmaster's badge of honor, an assurance their 'cue was smoked (never boiled) and cooked over a real Lipstick on a pig, so to speak. But sometimes with great beauty comes great danger. Most smoke rings are a direct consequence of meat sweating for hours in a toxic atmosphere of carbon monoxide. The same chemistry that dyes meat red, can turn a human into a pink-skinned corpse. Ninety percent of meat's red color reflects the myoglobin stored in every muscle cell. Like hemoglobin in blood (which drains completely away after butchering), myoglobin is red due to an iron molecule. Think "rust", a different form of an oxidized red iron molecule. As meat is heated, the myoglobin molecule starts to break apart, and between 145F-175F myoglobin turns permanently gray. The color of well-done meat. But, if the molecule is exposed to carbon monoxide before reaching this "denaturing" temperature, the CO molecule attaches to the iron (carboxymyoglobin and denatured CO hemochrome), stabilizing the pink color to well above 240F. Because CO gas penetrates from the outside in, and the CO molecule is relatively mobile in meat or fat, a pink ring is created. But first you need a source of carbon monoxide. Now it turns out a hot, well mixed and fully oxygenated fire produces only CO2 and water, which will not color myoglobin. For example, gas grills almost perfectly convert propane into water, carbon dioxide and heat. So there is little to no smoke ring on a gas grill1. Electric heating elements do not burn carbon fuels at all, so they also do not produce a smoke ring. But wood and charcoal fires burn carbon incompletely, producing high levels of carbon dioxide AND carbon monoxide, along with a robust smoke ring. The combustion temperature also matters- as the temperature rises, more CO is generated. Carbon embers speed up ("catalyze") the conversion of carbon dioxide to carbon monoxide. This reaction starts around 800F, and by 1200F more than 50% of the carbon oxides are CO. Almost every wood fire I've measured- a charcoal smoker, an offset, even an electric smoldering wood chips, generates more than 5,000 ppm of CO. Yet not every smoker with CO levels this high, exhibits a smoke ring. Because it fades away.... At body temperature, carbon monoxide bonds 200x more strongly to blood (and 50x more strongly to myoglobin) than oxygen, so after a burst of carbon monoxide exposure, it takes hours for the CO to dissipate and be replaced by oxygen. As a result of this long clearance time scale, your blood is constantly sopping up the small (~10 ppm) chronic CO levels found around gas appliances or from air pollution. Perhaps it's not too surprising to learn that 0.5% to 1.5% of normal blood and muscle cells are typically contaminated with CO2. Fortunately, blood (hemoglobin) and meat juices (myoglobin) are designed to purge excess CO in the presence of oxygen. Over time, purging by breathing air comes into equilibrium with low level CO exposure, and prevents the 1% from rising any higher. Good thing, too. If a kerosene space heater is unvented, or a gas water heater flue blocked, death from carbon monoxide poisoning happens all too frequently. When exposed to low levels of carbon monoxide, a few hours in a 100% oxygen tent can restore blood to normal levels. But higher, acute exposure conditions are difficult to shift. When 20% of your blood oxygen is replaced by CO, you become nauseous and dizzy. At 40% comatose. And die at 60%. Even those fortunate enough to survive, are often saddled with lifelong physical and neurological damage. This low/acute CO exposure threshold is also evident in smoke rings. In this piece of brisket, exposed to 25 ppm CO gas produced chemically and cooked to 180F in a 300F oven, only a light brown stain appears as a ring, and the stain likely marks the boundary between oxy and deoxy myoglobin:
On the other hand, a near permanent nitric oxide ring would appear at 25 ppm of NO. Why the difference? One clue can be found by eliminating oxygen from the exposure test. This hunk of brisket was immersed in 3% carbon monoxide and 99% nitrogen at 225F until the center reached 180F. Sliced, a classic red smoke ring is revealed: 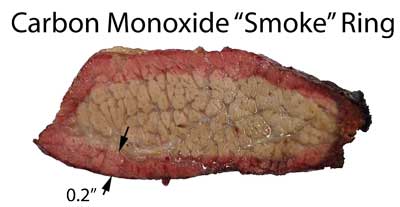
Brisket sample exposed to CO for 3 hrs at 225F. But when you slice the meat and expose it to oxygen (just like treating a comatose patient in an O2 tent) the CO is slowly displaced in favor of oxygen2. This time-lapse movie demonstrates how a slice of brisket cooked in an electric oven with a high CO level, fades from pink to gray over the course of 30 minutes. Note the surface is already dark gray- from oxygen entering the meat before slicing: Dry wood is 50% carbon and only 0.25% nitrogen. So carbon monoxide levels in wood fires are almost always 100 times greater than nitrogen oxide levels. Thus all smoke rings ought to be carbon dioxide rings. But it turns out myoglobin-absorbed NO is much more stable than CO, especially after heating above ~160F. And thus responsible for the ring. While it is true lower levels of CO can "pink" light colored meats (especially if permitted time to diffuse inward during storage), at high cooking temperatures, with competition from water and oxygen, CO levels greater than 1% are needed to form a deeply colored ring. Carbon monoxide rings do occur, but often fade away during the hours sweating in the Texas Crutch, or after slicing. Leaving the much stronger and more stable nitric oxide ring to paint the pig red. Most smoke rings are not really associated with the flavor and aromatic chemicals we think of with barbecue, but from incompletely burned carbon and nitrogen. This, plus the ease of faking a pink ring with sodium nitrite in a rub, is why smoke rings do not count in competition. Except everyone knows they are critical to appearance, and we eat with our eyes as well as our nose and tongue. So learning how to reliably create an attractive pink smoke ring is one crucial step towards mastering the barbecue.
|
||
|
1 Gas smokers are never perfectly efficient, so produce tiny amounts of CO. Just enough to noticeably pink a white meat like chicken or turkey. But the color fades pretty quickly on slicing. A few gas smokers generate as much as 20 ppm of nitric oxide by breaking apart the nitrogen in air. This gas produces an easily visible ring in brisket. Somedays. 2 CO bonds 50 times more strongly than oxygen to myoglobin. But the bond is not permanent. It is constantly gaining and losing this iron partner. So myoglobin, surrounded by an equal amount of oxygen and CO, eventually becomes saturated with CO. This accumulation process explains why 20 ppm CO in smoke can become 10,000 ppm in myoglobin. In other terms, constant exposure to 400 ppm CO will result in 10% blood CO, and hospitalization. But if there is no CO in the surrounding air, the CO reluctantly migrates out of the myoglobin to be replaced almost entirely by oxygen. Even in a CO-free environment, your body produces CO as a by-product of recycling worn blood cells. This is responsible for a 0.5% CO baseline in most healthy people. Smokers or workers in high pollution environments suffer from levels closer to 4%. |
||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |
 wood fire. A bright red smoke ring turns a gray, mundane slice of meat into an colorful, fun, attractive bite.
wood fire. A bright red smoke ring turns a gray, mundane slice of meat into an colorful, fun, attractive bite. 