| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| nitric oxide smoke ring | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
May 2014 Summary nitrogen oxide smoke ring:
Nitrogen: We swim in a sea of nitrogen-laden air; Yet nitrogen is annoyingly difficult to extract from the atmosphere. With apologies to Coleridge:
The nitrogen-nitrogen bond is extremely strong. To break this bond apart requires a temperature higher than 2900F- much higher than available in a wood fire1. This strong bond is one reason why many explosives gain their impact from breaking apart a nitrogen compound- most famously TNT (trinitrotoluene), but sadly, also fertilizers like ammonium nitrate. There are natural events strong enough to do the job. Every time a lightning bolt strikes, nitrogen is ripped out of thin air, turning N2 into nitrous oxides (NOx), which are easily absorbed by living plants and animals. Once incorporated in living creatures, these oxides are constantly recycled as plants live, die and rot. While air is 78% nitrogen and lightening strikes 100 times a second, electrical discharge is not the primary source of nitrogen oxides. Instead, a small group of microbes have evolved the rare capability to remove (e.g. "fix") nitrogen from air. Many plants symbiotically provide homes for these micro-organisms, housing a captive nitrogen fertilizer factory within their roots. The most humble of creatures makes abundant life possible. In trees, nitrogen is mostly deployed in leaves as a catalyst to speed up important chemical reactions, like photosynthesis. Wood trunks are primarily cellulose and lignin, which do not contain nitrogen. Why waste this scarce resource on mere scaffolding? So nitrogen is unevenly distributed throughout the plant, for example:
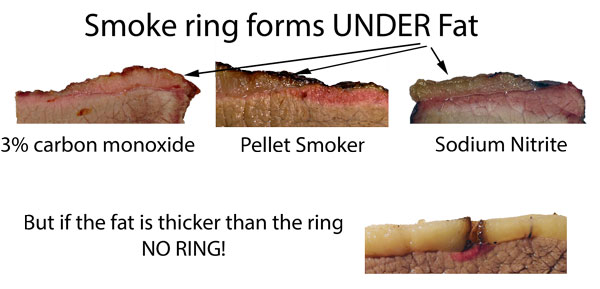
When organic matter is compressed over millions of years into oil or coal, nitrogen levels of 1% -2% accumulate. Grasses, leaves and nitrogen-fixing plants can reach as high as 4% nitrogen! This nitrogen is loosely bound to other organic molecules in the fuel, so unlike nitrogen in air (N2), can be released at much lower temperatures- even a few hundred degrees Fahrenheit. The science of combustion is complex and specific to the fuel and furnace in which it is burned. But there are a few common themes. First, most smoker and grill fires are deficient in oxygen, running coolly and unevenly. So they produce hundreds of fragrant and toxic organic chemical compounds, rather than a few simple byproducts like water and carbon dioxide. Dozens of nitrogen compounds are released during combustion- most notably nitrogen oxide (NO). The higher the temperature, the more NO produced. The greater the nitrogen content of the fuel, the greater the percentage of nitrogen compounds released. After combustion, the gases mix and react further. For example, nitrogen dioxide (NO2) and water (H2O) may combine into nitric acid (HNO3). But research and field measurements indicates the vast majority of nitrogen oxides released by combustion are in the form of NO (nitric oxide), with much lesser amounts in other configurations. In total, nitrogen oxides make up 0.02% of wood combustion byproducts. But this small amount can produce large visible effects. Ninety percent of meat's red color comes from myoglobin. Like hemoglobin in blood (which is completely drained out of meat after butchering), myoglobin is red due to an iron oxide molecule within the myoglobin. Think "rust", another oxidized red iron molecule. As meat is heated, the myoglobin molecule starts to break apart, and around 160F-170F myoglobin turns permanently gray. The color of well-done meat. But, if the molecule is exposed to nitric oxide before reaching this "denaturing" temperature, the NO molecule attaches to the iron (nitrosomyoglobin), stabilizing the pink color to well above 240F. Since NO gas penetrates from the outside in, a pink ring is created. Nitric oxide is a compact an almost neutral molecule, which means it easily diffuses through fat and water-laden meat2. In just a few hours the NO molecule can travel half an inch or more, leaving behind a wide, pink ring.
But not all barbecue fuels contain nitrogen, and even when they do, nitric oxide is not always formed by combustion. The NO content (as measured in actual fires or smokers) varies wildly, and in parallel to the reported intensity and reproducibility of the smoke ring:
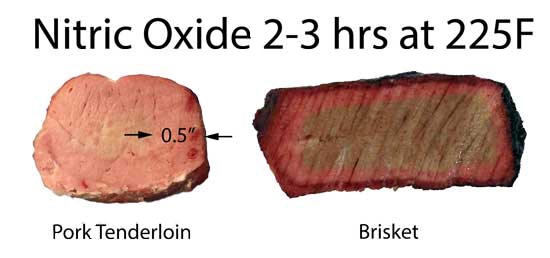
(for more details on specific smokers and measurement conditions, click here) Gas grills almost perfectly convert propane into water, carbon dioxide and heat. So there is little or no smoke ring on a gas grill. Electric heating elements do not burn carbon fuels at all (but of course they may at the generating station), so they also will not produce a nitric oxide smoke ring. Even when you add wood to an electric smoker- the combustion temperature and oxygen supply is simply too low to synthesize NO. We built a simple gas dosing rig, exposing pork tenderloin and brisket to bottled nitric oxide at the 1-3% level at 225F. Once the meat reached 180F internal it was removed from oven and sliced open. Clearly, pure NO is able to produce a robust "smoke" ring:
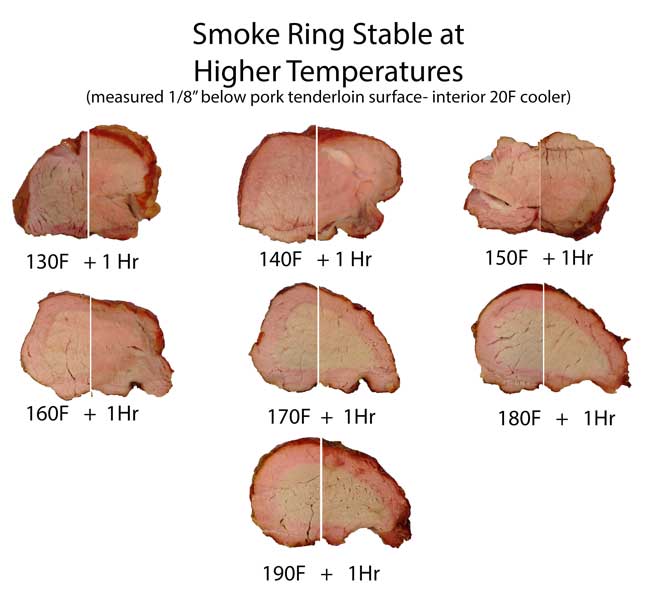
Pork and Brisket exposed to NO and air in closed humid flask In a real smoker, two gases contribute to the smoke ring: carbon monoxide and nitric oxide. The carbon monoxide levels are typically a hundred times larger than the nitric oxide, but the carbon monoxide ring tends to quickly fade away in the presence of oxygen and nitric oxide. Oxygen, because it outnumbers carbon monoxide, and nitric oxide, because it bonds more aggressively to myoglobin. Either way, it knocks the CO out of the myoglobin iron complex. Nitric oxide smoke rings are much more stable than CO, but only after the temperature rises high enough to convert one pink color of myoglobin (nitrosomyoglobin) to a more stable, darker red form. There is some debate over the exact chemical make-up of this more stable red color- it may be nitrosylhemochromogen, or a one of the denatured hemochrome family where both carbon monoxide and nitric oxide combine to rearrange the iron ring. There is even some evidence two NO molecules can bond to the iron ring at high temperatures. In either case, compared to the quick fade of a pure carbon monoxide ring, a nitric oxide ring will outlast dinner and the next day's leftovers. In this split screen montage we illustrate how the smoke ring on a pork tenderloin fades away in an hour if the ring's temperature is below ~170F, but is stable for hours (or days if cold) above 170F.
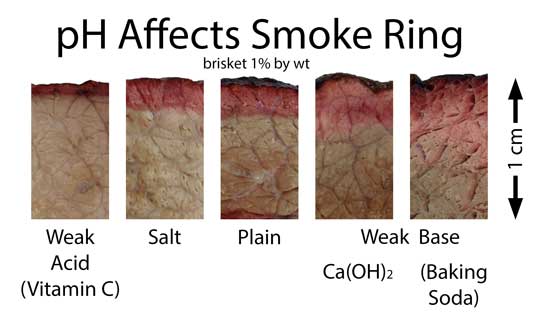
(similar higher resolution images, including a more stable ring in brisket where the myoglobin levels are greater and of a different chemical configuration, here) Many backyard cooks and even competition pit-masters despair when the ring sometimes fails to appear, even though their technique was seemingly unchanged from cook to cook. There can be many reasons for this "Cheshire cat" effect, but chief among them is the fuel nitrogen content and meat pH. As the above table indicates, some fuels and smokers deliver less than 20 ppm of NO, and thus a weak ring. If the wind conditions reduce airflow across the wood, or that batch of lump charcoal is from a low-nitrogen tree, the NO levels many drop below 10ppm, and the ring will be absent. Another day, another batch of wood, and the ring reappears. pH is also a critical factor. Meat pH typically runs from 5.2 (very low) to very high (6.8) due to slaughter and aging conditions, feed, species, etc. High pH stabilizes myoglobin from falling apart in the heat, while low pH drops the graying temperature by up to 20F. High salt will also slightly depress myoglobin stability. The longer myoglobin can resist "denaturing", the longer time the ring has to grow. To illustrate the pH effect, I rubbed briskets with weak acid and weak base powders. The acid dramatically reduces the smoke ring thickness, while the bases almost triple its width compared to untreated meat:
Since wood smoke itself is often acidic, controlling surface pH with the right rub and mopping process, can be critical. There is some controversy over the exact series of chemical reactions that transports nitrogen from the fire and to the meat- I discuss a few of my own experiments in the footnote below2. But for most of us, the simple and accurate explanation for the barbecue smoke ring is:
|
||||||||||||||||||||||||||||||
|
1 These high temperatures may be reached in carbon-burning power plants- whether natural gas, coal or wood. Nitrogen compounds released by heating air intensely are then chemically modified by light and ozone into smog. Even more smog is produced if the fuel itself contains nitrogen. Natural gas is free from nitrogen, but coal levels may be as high as 1.5% and grasses (whose leaves are nitrogen rich) often reach 4%. Making their continued or future role as a renewable fuel problematic. How much of this nitrogen ends up in the flue gas is easy to guesstimate. Let's say dry wood contain 0.25% nitrogen (2500ppm). Because we burn wood with air and not pure oxygen, and oxygen makes up only 21% of air, this dilutes the exhaust gases by a factor of 5, down to 500ppm. But then most wood combustion is not complete. We've measured the fraction of oxygen consumed in many smokers, and it runs from half to 80%. With perfect combustion there would be no remnant oxygen. In other words, much of the air simply bypasses the fire, or is only partly combusted. Another factor of say, 3. So the maximum nitrogen level we might expect from a wood fire in a smoker is around 150 ppm. In the ballpark of our measurements. Some gas smoker flames reach 2300F or so. Which is not hot enough to break apart every nitrogen air molecule, but will disassociate 1 out of every 25000, leading to NO flue concentrations of 20 ppm or below. Which is just high enough to result in a smoke ring without the need to add wood. Sometimes. 2 Nitrogen oxide production is very complex. In the intense heat and light of the flame, many compounds are created and destroyed in microseconds. Others morph into new configurations on their way out of the fire and into the water pan. And still others make it all the way to the meat's surface intact. So it's hard to generalize, especially in smokers which are barely studied by the academic community, but let's throw caution to the wind, and give it a whirl. By combusting the few tenths of percent of nitrogen present in wood or coal, fire produces NO, N2O, NO2, HCN, NH3 and hundreds of other compounds. The ratios depend strongly on temperature and oxygen levels. Hydrogen cyanide (HCN) and ammonia (NH3) occur at lower temps and help catalyze the formation of NO in the flame, so they are found in limited quantities downstream- providing there is enough oxygen. Of these downstream chemicals, the most stable and prominent is nitric oxide (NO). Its hydrated form, nitrous acid (HNO2) is extremely fragile. In fact, nitrous acid is normally stored in lab on ice, or it will spontaneously decompose into NO at room temperature. So its very rarely detected in cooled combustion streams. Among the many hazy rumors surrounding the details of the NO ring, a common belief asserts smoke rings form only below 140F on moist salted surfaces. Once meat is heated above this temperature, the story goes, no additional smoke ring growth is possible. A complex "just so" story....
But there are a number of problems with this story, which we also discuss in our article on moisture and the smoke ring. The pioneering Cornforth gas oven experiments were performed at NOx levels typically below 1 ppm, and CO levels typically under 10 ppm, and then *presumed* by others to apply in a smoker. But a wood or charcoal smoker generates 10 - 100 times greater concentrations than a gas flame, and since both oxygen, carbon monoxide and nitric oxide compete to fill myoglobin's iron ring, concentration tips the balance of these reactions away from those in the original paper. Other differences include
While the gas oven experiments confirmed that drying the surface eliminated the ring, this is NOT evidence that moisture is a necessary part of the chemical reaction pathway. Instead, we've demonstrated that dry surfaces simply attract fewer gas molecules from the smoke. Again, a concentration issue. We also looked at nitric acid as a possible ring intermediate step: At 575F, nitrogen dioxide's (NO2) half life conversion to NO and oxygen is around 5 minutes- so a fraction will remain intact from fire to meat. And it will form nitric acid in contact with water. Nitric acid HNO3 is problematic. The acid can in principle can break nitrate (NO3) down into nitrogen dioxide, and then into NO. But in practice the acid and nitrate are remarkably stable. Sausage makers are aware of this stability- they use Prague Salt #2 to cure meat. Prague salt #2 contains both sodium nitrite, which immediately turns meat pink upon cooking, and sodium nitrate, which breaks down into nitrite over MONTHS. The long conversion period assures the nitrate predictably turns into nitrite and thence into NO, continuing to preserve sausage as it dehydrates and cures. We confirmed this stability in a few experiments applying nitric acid directly on meat. For example, we slowly simmered pork tenderloin in 5M nitric acid, raising the temperature to 200F over 3 hours. The surface turned yellow (the xanthoproteic protein reaction), the meat fell apart, but a small ring emerged. We also dipped pork tenderloin and steak in nitric acid for 15 minutes , then baked at 325F until the interior was gray and fully cooked. Again a stable pink ring appeared on the pork, but only a gray-green ring on the beef.
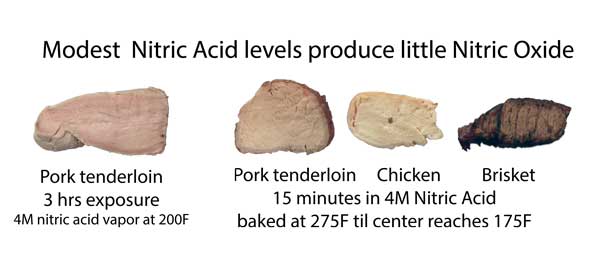
However, these level are unrealistically high. If we back slightly off, down to 4M (still insanely high) and try lower temperatures typical of smoking, immersed or in vapors, the ring never appears:
If we had applied sodium nitrite directly at the same ionic levels, the meat would be bright pink. Many writers assume, that since the acid-to-nitric oxide reaction is possible, it is also probable. Or they are familiar with textbook nitric acid synthesis pathways, and assume similar chemistry applies inside a smoker. But that is not the case.
|
||||||||||||||||||||||||||||||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |