| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| wood fuel | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
May 2014 Wood Fuel Summary:
360 million years ago the first trees appeared in the fossil record. As trees emerged at the beginning of the Carboniferous period, evolution had a clever trick up its sleeve - they were infused with lignin. A complex organic molecule nearly indigestible by all but a small class of fragile fungi and bacteria, lignin grants trees structural strength and resistance from rot. Good thing too- had some other microbe discovered the secret to digesting lignin, our forests would be reduced to a slimy green goo, our houses dissolve away, and most important of all, we wouldn't have barbecue. The trees also filled our atmosphere with oxygen- nearly doubling its concentration- and without oxygen, we wouldn't have fire. No grilling and no barbecue- hardly worth the trouble of evolving hands and a big brain... When trees die, this rot resistance allowed them to accumulate in great numbers over the eons. With time and pressure, old trees trunks and other organic matter transformed into new coal. So when you cook with coal, you are cooking with wood. While modern trees have evolved in complexity, fundamentally they are nothing more than big candy canes dipped in lignin. Why candy? Well, green wood is at least half water. The remaining fibrous parts are sugars (the indigestible carbohydrates cellulose 40%-60%, and hemi-cellulose 20%-30%) and lignin 20%-30%. Plus trace levels of minerals such as potassium, sodium and calcium salts and oxides whose concentrations reflect local soil conditions. In terms of chemical elements, an average dry tree trunk consists of:
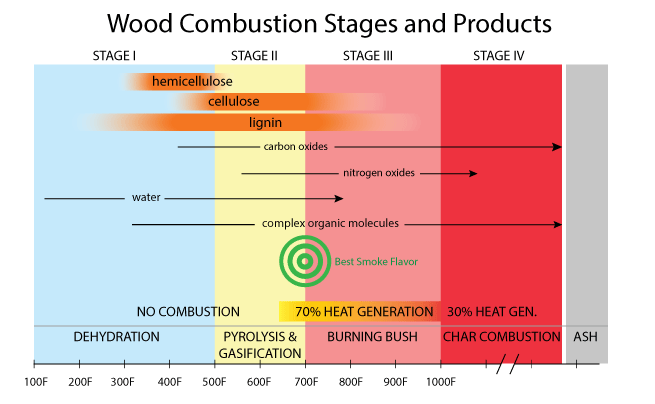
Trees convert sunlight into food by photosynthesis, and that food is the simple sugar glucose. With glucose as a "brick", trees are efficient masons, building their primary structural walls out of a chain of dried-out glucose molecules- that is Glucose - Water = One Cellulose Brick C6H12O6- H2O=C6H10O5 Compared to cellulose, water-proofing rot-resisting insect-repelling lignin is by far the more complex molecule (and still not completely understood). Lignin contains bound aromatic phenylpropane alcohol molecules1 in its structure which helps to stabilize the cellulose chains. If cellulose is the bricks and mortar of wood, lignin is the steel rebar and foundation waterproofing. When burned, lignin breaks down into most of the molecules we associate with smoke color and flavor. And how does wood burn? Well, incompletely and unevenly. Incompletely, in the sense that chemically wood COULD combust into just water and carbon dioxide and nitrogen gas and some mineral ash, if there were sufficient oxygen and agitation. Such complete combustion is only possible in a power station furnace fanned by compressed air or pure oxygen, with a fluidized bed to keep the embers agitated. But never in a fire. This is because wood burns sequentially. Wood is a good insulator. It can be flamingly hot on one end and cool enough to grasp on the other. The inside of a log might be wet (even split dried wood is still 15% or more water) while the surface is in embers. For this reason, a wood fire is never at a single temperature. Wood combustion passes through four stages, which we will describe as if the stages were distinct, but again in a real fire or inside a barbecue smoker, all four stages occur simultaneously. For this reason I've rounded some of the temperature bands to reflect this essential uncertainty, and to avoid the trap of false precision. And remember, "temperature" is not an average across the entire bed of embers, but refers to the local conditions right at the surface, following the combustion of one small section of wood. In addition, the chemical byproducts of wood combustion are not a universal constant, but depends on the tree species, its age, and even its mineral composition. These minerals, like sodium and calcium salts, act as catalysts and can shift the relative abundance of certain combustion products significantly. Finally, the compounds deposited on your meal have been re-combusted and recombined on their way from the fire to the food. Exactly which compounds dominate is a function of the smoker's design, and the management of the embers. Wood smoke contains many dangerous, albeit useful chemicals. The same compounds in lignin that prevent wood rot, emerge in combustion as wood creosote which is a powerful anti-bacterial and food preservative. This, along with the drying action of a hot fire, helped early man preserve food for winter. Smoke also contains poisons like carbon monoxide, carcinogens like formaldehyde, and smog catalysts like nitric oxide. One reason wood stove emissions are tightly regulated- a bit of barbecue smoke on the weekend is one thing, but a city filled with pellet furnace smoke is another.
Stage 1: Dehydration (below 500F) Up to about 300F water is steaming away, the wood is drying out, and finally some simple molecules like carbon dioxide2 and methanol emerge. No flame, and no heat. Thus an external energy source is required to pass through the Dehydration stage- in a barbecue, usually heat from a burning newspaper, lighter fluid, or even a blow torch to get the coals started. Stage 2: Pyrolysis and gasification (500F to 700F)- Greek for "lacking fire", pyrolysis causes organic molecules to breakdown into new compounds, but the lack of oxygen means these compounds won't burn. Pyrolysis typically occurs inside wood or near the surface- both areas deficient in oxygen, creating oily compounds and some volatiles. Gasification is a similar process outside the wood- the pyrolytic compounds morph again into vapors or droplets and flow away from the wood. Hemicellulose first decomposes into acids and ammonia and volatile gases, then cellulose into more gases and tars, and finally lignin into a broad spectrum of aromatic chemicals. Near the top of this temperature range, these gases will burn if exposed to a flame, but not self-ignite. Stage 3: Burning Bush (700F-1000F). Later stage, secondary pyrolysis. Combustible gases, like methane, carbon monoxide and hydrogen emerge and can ignite. They burn even while the underlying wood simply chars, but does not combust. Sort of like a candle wick, which burns for a long time without being consumed (in fact, the blue flame at the bottom of a candle wick is burning carbon monoxide). Hydrogen cyanide and ammonia (NH3) are now converted to nitric oxide (NO), providing there is sufficient oxygen. Aromatic smoke flavors emerge in this stage- and peak in desirability between 650F and 750F. Above 750F, the lignins remain aromatic, but are reduced in quantity and new, toxic chemicals are produced. Stage 4: Charcoal (above 1000F). The wood is now almost completely carbon, and known as "char". Most of the complex organic molecules have evaporated or burned away. With enough oxygen, charcoal combusts at a temperature greater than 1500F with little odor or smoke. As a backyard cook or pitmaster, your job is to simultaneously manage combustion products and temperature. And herein lies a conundrum. In most smokers, you add fuel and then adjust the air vents to control temperature. The fuel finds its own equilibrium between these four stages. Sometimes that equilibrium creates a light sweet smoke flavor, and sometimes a dark gray sooty fog. In principle, you could add a small amount of fuel, and a proportionally small amount of air. The small amount of fuel provides just enough energy to keep the smoker running low and slow, while the correct amount of air maintains proper combustion conditions. For example, a pellet smoker. A pellet smoker is a bit like a miniature, efficient power-station furnace. Small quantities of compressed sawdust pellets are dropped in a cup. A fan blows air into the cup and a hot wire ignites the wood. Small fuel, proportionally small air. In principle perfect combustion, and adjustable to hit the "best smoke flavor" temperature target. Unfortunately, the same combustion gases that flavor food are used to heat the smoker, and that heat demand varies in time. As the fan is cycled on and off to maintain a low and slow temperature, combustion remains incomplete through much of the cycle, especially while reigniting. Conversely, when pellet smokers are set to run hot and fast, the combustion chamber remains lit and furious- replacing all the air in the cooker 300 times in an hour! Combustion is so complete, people complain the smoke has no flavor.
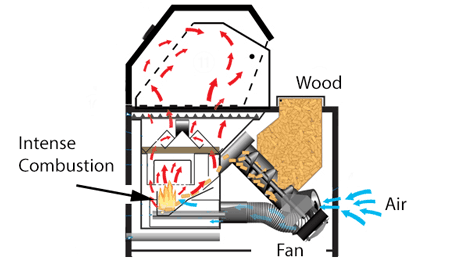
This trade-off could be avoided in a pellet smoker designed to constantly run the fan and fuel at optimal levels, with a mechanical diverter to send hot combustion gases to the smoking chamber as needed. Or, you could separate the smoking and cooking functions- for example, heat the meat with propane and, by attaching a small external pellet smoker, admit ideal smoke with little heat. Most large commercial smoke houses operate with independent systems for heat, smoke and humidity. You don't actually need the wood to burn with an open flame! Simply placing the wood pellets in an insulated box heated externally to 700F will pyrolized more than enough smoke flavor molecules (but will not produce enough nitric oxide for a smoke ring). This is more or less what a wood chip foil pack, tossed on a gas grill, is trying to simulate. I've measured the gas emissions from a number of smokers, and most reduce the exhaust oxygen level from 21%(incoming) to 19%(outgoing). With complete combustion the exhaust oxygen would be reduced to near zero- so in fact almost 90% of the incoming air bypasses the fire. High efficiency home pellet stoves may drop oxygen levels to 13% instead of 19%, and consequently emit far fewer pollutants. They do this by directing the incoming air into a separate pellet combustion chamber, and preheating the supply air with the hot exhaust. However, the vast majority of backyard smokers adopt a much simpler approach- a single chamber containing fire, wood, meat and smoke. Within the shared chamber, a tray is filled with lit fuel, while sliding vents and chimney dampers control ventilation and temperature. This design is common in ceramic smokers, cabinet smokes, gas smokers, ... One common mistake is to add too much fuel, and then be forced to throttle back oxygen flow to maintain a low temperature. In this case, the combustion reaction drives towards early Stage 2, and the chemical balance shifts toward tars and soot. Leading to common complaints about "bitter, over-smoked" meat. To try and bring the fuel/air mixture back into balance, many people arrange their wood in the shape of a long, sinuous maze lit from one end (the "minion" method), so only a few hunks are burning at one time. Which definitely extends cook time. It also lowers the temperature by reducing the size of the fire, rather than choking air from a large tray of barely lit embers. But combustion air flows over the entire ember bed, bypassing most of them, so the burning edge is often still oxygen starved. Lots of air, potentially, but very little reaches the fire line. A smoker which drops the wood next to the vent, in small quantities at a time like some gravity fed smokers, can burn more efficiently since the air is where it is needed most. Unfortunately, the average smoker has a primitive vent and fuel arrangement.
A second "trick" most experienced cooks know is to open one of two firebox vents all the way, and control the smoke chamber temperature with the chimney damper. In some wind conditions or with an electronic controller, air might flow from the left-side vent to the open right, keeping the fire burning. Heat cannot enter the smoke chamber because air flow is blocked by the chimney damper. This technique is particularly effective in reverse-flow cabinet smokers which limit convection via their switch-back air plenums. Not as effective in smokers with one large open chamber and tons of air leaks, or on a windy day, .... Another solution is to use a "burn box" to per-ignite wood before adding to the smoker. In this case Stage 1 and part of Stage 2 takes place outside the smoker. So when you add wood embers, you are only adding sweeter aromatic chemicals- not gray sooty smoke from the initial stages of combustion. Also, with a burn box you partly control the temperature with the amount of embers shoveled in- thus you can leave the vents open to oxygenate the fire. However, with this approach you are always tending the fire. Lack of oxygen is not as much as an issue for lump charcoal when used purely for heating- the volatile components were removed during charcoal production. But, if you toss a few hunks of wood onto the charcoal for flavor, make sure the vents are open during this stage, or risk tarry flavors. Wood, like charcoal, contains about 9000 BTUs of energy per lb, but is rarely uniformly combusted, so comes closer to 7000 BTU per pound. To fully combust this much fuel, you need to supply around 1 cf/hr of air flow for every 50 BTU/hr consumed3. Anything less, and the wood may smolder rather than burn. With this much airflow, an offset smoker might exhaust its entire volume more than 50 times an hour. This can dry out the meat without the addition of a large water pan placed near the fire, or constant mopping. Restaurants and commercial smokers often have so much meat cooking in their smokers that the meat drippings themselves are a significant source of moisture. While many recipes suggest moistening wet wood chips to control temperature, in practice this creates a lot of smoke (good) with bitter notes (bad). We discuss the flavor impact in other tabs, but soaking wood is a practice I avoid. Not all smoke is created equal. Because low temperature combustion produces acrid, sooty flavors, while high temperature combustion produces lighter flavors, it is a good practice to err on the side of higher temperature combustion. In other words, while a fully-agitated well-oxygenated fire running around 800F is ideal, in practice a 1400F dark red ember assures the cooler sections of the fire are still above 650F, so off flavors are avoided.As to seasoning, green wood is so wet that more than a third of the heat generated in a fire is simply wasted steaming out the moisture. And wet wood smolders instead of burning. So never smoke with green wood- let time and heat and air flow season split wood until it partially dries out. How dry? Well, there are two kinds of water in wood- "free" water, and "chemically bound" water. Like a sponge, free water is held inside pores by capillary action. Most of the pores in wood consist of long parallel tubes called xylem, which carry water from the roots to the leaves, and phloem, which carry sugars made by photosynthesis in the leaves, back into the rest of the tree. In green wood these tubes are filled to the brim with water. You can wring out this free water by air drying, which may take as little as a few days or weeks. Similar to wring out a sponge. But chemically bound water is more tightly attached to the wood molecules. Bound water is the moisture that remains in a sponge, even after squeezing in a vise. And will take longer to dry. One way to quantify wood moisture is the ratio of water (both free and bound) to the bone dry weight of wood. Or Moisture Content Dry MCD. Green wood in the spring may contain 1.5 lbs of water for every 1 lb of dry wood, or an MCD of 150%! Once the free water evaporates from the ends of the split wood (where the phloem and xylem tubes are exposed by the chainsaw), the MCD drops to around 30% (depending on species). The bound water then slowly dissipates. But never fully- wood will reabsorb moisture from the surrounding air. In an air conditioned home at 30% relative humidity, wood (after months or years) will come into equilibrium and dry out to around 6% MCD. Outdoors, in the humid South but protected from rain, split logs will drop to 15% MCD. But this is still too dry for smoking! Very dry wood burns so vigorously, it requires enormous amounts of airflow to keep up with the combustion process. In most smokers, the firebox's vent and doors limit this airflow. A low MCD fire burns intensely with strong air currents that propel soot and ash into the flames and onto the food. Nasty. But wood with 20%-25% moisture levels burns at just the right rate- not too fast, not too slow. I like to think of this as a candle wick. A short wick glows dimly and may burn out. A long wick creates a huge, sooty flame. But a medium wick glows brightly with no visible smoke. The best way to measure MCD is with an electronic moisture meter. These are inexpensive and accurate- but make sure to follow the manufacturer's directions. Old hands at smoking can roughly judge MCD by sound (tap on wet wood and it will make a dull thunk, bone dry wood will ring), or heft, or by the amount of cracking. Since every smoker is different you may find 27% is best for you, while your neighbor prefers 20% In a charcoal or gas smoker where small wood hunks are used for flavor, moisture still matters. These smokers tend to have very small vents, and often burn with too little oxygen for their own good. Bone-dry wood hunks from scrap oak flooring sometimes create sooty fires, while moist wood smolders. So even here MCD matters. |
||||||||||||||||||||||||||||||||||||||||||
|
1 Its easy to throw around long, sciencey sounding chemical names to make an impression. To bully people into thinking you are an authority. But for 99.99% of the readers of this article, myself included, the names are just more "blah blah blah". So I try to avoid unnecessary nomenclature where ever possible, while accurately conveying the underlying science. Sometimes knowing the name is important as a map to further learning, and for that reason important compounds will be mentioned in the body of the articles. But merely as signposts- for most readers, an alkyl is interchangeable with an aniline.
If interested, the EPA published a report on wood stove combustion products- I've average their results with other studies for this table. Consider this a rough guide, recognizing that different wood species and smoker designs will reorder the proportions. Note carbon monoxide makes up as much as 10% (100,000 parts per million) of the smoke, while nitric oxides are only 0.02% (200 ppm). For comparison, a home CO detector triggers around 70 ppm.
Chemical Composition of Wood Smoke
2 Also note that storing LARGE quantities of wood pellets or sawdust in a closed room on a summer day can produce carbon monoxide by oxidation of the fats in the wood. No flame required. The tiny amounts of CO from each pellet adds up, levels >1000 ppm have been measured and a few people have died entering pellet storage rooms. 3 One lb of wood requires around 10lbs of air for complete combustion, or (given air's density of ~0.07 lbs/ cuft), 150 cuft of air/ lb of wood. At 7000 BTU/lb of wood, this is ~1 cuft of air for every 50 BTUs.
|
||||||||||||||||||||||||||||||||||||||||||
Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |